Answer: Option i) Sodium carbonate is synthesised by Solvary ammonia soda process.
∆Uθof ignition of methane is – X kJ mol–1 . The worth of ∆Hθ is ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }=\text{ }U\theta \\ ~ \\ \left( ii \right)\text{ }>\text{ }U\theta \\ ~ \\ \left( iii \right)\text{ }<\text{ }U\theta \\ ~ \\ \left( iv \right)\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-253e16b0c605d0c940895437070f698c_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }=\text{ }U\theta \\ ~ \\ \left( ii \right)\text{ }>\text{ }U\theta \\ ~ \\ \left( iii \right)\text{ }<\text{ }U\theta \\ ~ \\ \left( iv \right)\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-253e16b0c605d0c940895437070f698c_l3.png)
Solution: Since \[\begin{array}{*{35}{l}} H\theta \text{ }=\text{ }U\theta \text{ }+\text{ }ngRT\text{ }and\text{ }U\theta \text{ }=\text{ }\text{ }X\text{ }kJ\text{ }mol1\text{ }, \\ ~ \\ H\theta...
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to (i) Ionic nature of lithium fluoride (ii) High lattice enthalpy (iii) High hydration enthalpy for lithium-ion. (iv) Low ionisation enthalpy of the lithium atom
Answer: Option ii) Lattice and hydration enthalpies encourage the dissolving of alkali metal halides in water. Fluorides are soluble in this order: LiF, NaF, KF, RbF, CsF. The high lattice energy of...
The order of decreasing ionisation enthalpy in alkali metals is (i) Na > Li > K > Rb (ii) Rb < Na < K Na > K > Rb (iv) K < Li < Na < Rb
Answer: option iii) Increasing the size of the atoms in a group results in a decrease in ionization enthalpy. As a result, the following is the ranking: Li > Na > K > Rb.
What happens when: (I) Sodium metal is drenched in water? (ii) Sodium metal is warmed in bounty of air? (iii) Sodium peroxide gets disintegrated in water?
Solution: (I) Sodium responds to shape NaOH and H2 gas when it is dropped in water. The response happens as displayed beneath: \[2Na\left( s \right)\text{ }+\text{ }2H2O\left( l \right)\text{...
Metals form basic hydroxides. Which of the following metal hydroxide is the least basic? (i) Mg(OH)2 (ii) Ca(OH)2 (iii) Sr(OH)2 (iv) Ba(OH)2
Answer: Option i) As the ionisation enthalpy increases from Mg to Ba, the M – O bond weakens and weakens down the group, and as a result, basicity increases down the group as a function of time. As...
Which of the carbonates given below is unstable in air and is kept in CO2 atmosphere to avoid decomposition. (i) BeCO3 (ii) MgCO3 (iii) CaCO3 (iv) BaCO3
Answer: Option i) Because of the smaller cation and larger anion sizes (smaller cation stabilises smaller anion through crystal lattice energy), Beryllium Carbonate can only be preserved in CO2...
Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally? (i) MgCO3 (ii) CaCO3 (iii) SrCO3 (iv) BaCO3
Answer: Option iv) The thermal stability of metal carbonates rises as the metal's electropositive character or basicity increases from Be(OH)2 to Ba(OH)2. The most stable is BaCO3.
Compose adjusted conditions for responses between: (a) Na2O2 and water (b) KO2 and Water (c) Na2O and CO2
Solution: \[\begin{array}{*{35}{l}} ~ \\ \left( a \right)\text{ }2Na2O2\left( s \right)\text{ }+\text{ }2H2O\left( l \right)\text{ }\to \text{ }4NaOH\left( aq \right)\text{ }+\text{ }O2\left( aq...
The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution. (i) Sublimation enthalpy (ii) Ionisation enthalpy (iii) Hydration enthalpy (iv) Electron-gain enthalpy
Answer: option iii) Li's hydration enthalpy is likewise high (highly exothermic). Li atom has the highest hydration enthalpy, making it the strongest reducing agent in aqueous media. - Li atom's...
What happens when (I) magnesium is scorched in air (ii) fast lime is warmed with silica (iii) chlorine responds with slaked lime (iv) calcium nitrate is warmed ?
Solution: (iii) When chloride is added to slaked lime, it gives blanching powder. \[Ca\left( OH \right)2\text{ }+\text{ }Cl2\text{ }CaOCl2\text{ }+\text{ }H2O\] Blanching powder (iv)...
Starting with sodium chloride how would you proceed to prepare (i) sodium metal (ii) sodium hydroxide (iii) sodium peroxide (iv) sodium carbonate?
Solution: (a) Sodium can be extricated from sodium chloride by Downs measure. This interaction includes the electrolysis of intertwined NaCl (40%) and CaCl2 (60 %) at a temperature of 1123 K in...
Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide. Reason (R): Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Answer: Option (i) is correct. Beryllium carbonate is stable and decomposes to give beryllium oxide and carbon dioxide. The concentration of carbon dioxide grows in the right side, causing the...
Match the compounds given in Column I with their uses mentioned in Column II.
Column I(i) CaCO3 (ii) Ca(OH)2 (iii) Cao (iv) CaSO4 Column II(a) Dentistry, ornamental work (b) Manufacture of sodium carbonate from caustic soda (c) Manufacture of high-quality paper (d) Used in...
Look at the solvency and warm dependability of the accompanying mixtures of the salt metals with those of the antacid earth metals. (a) Nitrates (b) Carbonates (c) Sulfates.
Solution: (I) Solubility: Soluble base metal nitrates, carbonates and sulfates have water solvency. At the point when you drop down the gathering of soluble base metals, you will see that the...
Talk about the different responses that happen in the Solvay interaction.
Solution: We realize that the Solvay interaction is utilized in the readiness of sodium carbonate. This interaction is financially savvy contrasted with different cycles of arrangement of Sodium...
Discuss the trend of the following: (i) Thermal stability of carbonates of Group 2 elements. (ii) The solubility and the nature of oxides of Group 2 elements.
Answer: i) Carbonate thermal stability rises with cationic size. The more stable an alkaline earth metal's oxide, the less stable its carbonate. As BeO is stable, BeCO3 is not. (ii) Alkali metals...
In what ways lithium shows likenesses to magnesium in its compound conduct?
Solution: Likenesses among lithium and magnesium: (I) lithium and magnesium respond delayed with cold water. (ii) oxides of lithium and magnesium are less dissolvable in H2O. additionally the...
Name an element from Group 2 which forms an amphoteric oxide and a water-soluble sulphate.
Answer: Beryllium is from Group 2. Unlike the other chemicals, beryllium oxide is amphoteric. Group 2 sulfates are water-soluble, as is $BeSO_4$.
Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Answer: i) In terms of weight and hardness, lithium and magnesium are both significantly lighter and tougher than the other metals in their respective families. (ii) Both LiCl and MgCl2 halides are...
Complete the following reactions (i) 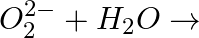 (ii)
(ii) 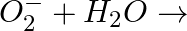
Answer: (i) Peroxide ions react with water and form $H _{2} O _{2}$. $O _{2}^{2-}+2 H _{2} O \longrightarrow 2 OH ^{-}+ H _{2} O _{2}$ (ii) Superoxides react with water and form $H _{2} O _{2}$...
2.9 g of a gas at 95 °C involved a similar volume as 0.184 g of dihydrogen at 17 °C, at a similar tension. What is the molar mass of the gas?.
Solution:
When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K.
Answer: The reactivity of alkali metals towards oxygen rises with atomic size. So Li only produces LiO2 (Li2O). Sodium forms mostly sodium peroxide and a little sodium oxide, while potassium forms...
How do you account for the strong reducing power of lithium in aqueous solution?
Answer: Lithium has the largest negative E value of any element, measuring –3.04V. Lithium has tiny atomic size and the highest ionization enthalpy of all the elements, although this is offset by...
What will be the strain applied by a combination of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm3 jar at 27 °C?
Solution: we know that,
sing the condition of state pV=nRT; show that at a given temperature thickness of a gas is corresponding to gas pressure p.
Solution: The condition of state is given by, \[\begin{array}{*{35}{l}} pV\text{ }=\text{ }nRT\text{ }\ldots \text{ }..\left( 1 \right) \\ ~ \\ Where,\text{ }p\text{ }=\text{...
A uniform disc of radius R, is resting on a table on its rim. The coefficient of friction between disc and table is μ. Now the disc is pulled with a force F as shown in the figure. What is the maximum value of F for which the disc rolls without slipping?
Solution: Let the linear and angular acceleration be $a$ and $\alpha$ respectively. So, $F – f = Ma$ Where, M = mass of the disc f = force of friction applied at the centre Torque to disc,...
A uniform square plate S and a uniform rectangular plate R have identical areas and masses. Show that
a) 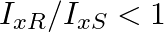
b) 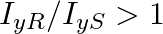
a) $\mathrm{c}^{2}=\mathrm{ab}$ as $\mathrm{l}=\mathrm{mr}^{2}$ $\frac{I_{x R}}{I_{x S}}=\frac{m \frac{b}{2}^{2}}{m \frac{c}{2}^{2}}=\frac{b^{2}}{c^{2}}$ Because $c>b$ we can say, $c^{2}>b^ 2$...
Two cylindrical hollow drums of radii R and 2R and of a common height h, are rotating with angular velocities ω (anti-clockwise) and ω (clockwise) respectively. Their axes, fixed are parallel and in a horizontal plane separated by (3R + δ). They are now brought in contact 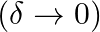
a) show the frictional forces just after contact
b) identify forces and torques external to the system just after contact
a) We know, $v_{1} = \omega R$ $v_{2}=\omega^{2}R$ The direction of $v_{1}$ and $v_{2}$ are tangentially upwards in the figure, and they meet at point C. As a result, $f_{12}=-f_{21}$ represents the...
A disc of radius R is rotating with an angular speed 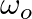 about a horizontal axis. It is placed on a horizontal table. The coefficient of kinetic friction is
about a horizontal axis. It is placed on a horizontal table. The coefficient of kinetic friction is 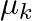 .
.
a) what happens to the linear speed of the centre of mass when the disc is placed in contact with the table?
b) which force is responsible for the effects in previous questions?
a) The linear velocity of the revolving disc changes as it is brought into contact with the table. b) Frictional force is responsible. The figure depicts it:
Two discs of moments of inertia 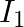 and
and 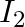 about their respective axes and rotating with angular speed
about their respective axes and rotating with angular speed 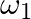 and
and 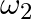 are brought into contact face to face with their axes of rotation coincident.
are brought into contact face to face with their axes of rotation coincident.
a) calculate the loss in kinetic energy of the system in the process
b) account for this loss
a) Final kinetic energy = rotational + translation energy $K_{f}=KE_{R}+KE_{T}$ $\Delta \mathrm{K}=-I_{1} l_{2} / 2\left(I_{1}+l_{2}\right)\left(\omega_{1}-\omega_{2}\right) 2<0$ b) Because...
Find the centre of mass of a uniform
a) half-disc
b) quarter-disc
Solution: Let the mass of the half-disc be M Area of the half-disc will be $\frac{\pi R^{2}}{2}$ Mass per unit area will be $\frac{2M}{\pi R^{2}}$ a) when the disc is half, the centre of mass is (0,...
A door is hinged at one end and is free to rotate about a vertical axis. Does its weight cause any torque about this axis? Give a reason for your answer
Solution: The door's axis is in the y-axis, and it is in the x-y plane. As the force is applied in the z-axis, the door rotates in both the positive and negative directions. Gravity is acting on the...
The vector sum of a system of non-collinear forces acting on a rigid body is given to be non-zero. If the vector sum of all the torques due to the system of forces about a certain point is found to be zero, does this mean that it is necessarily zero about any arbitrary point?
The torques' vector total equals zero. However, the net force is not zero. The following is the mathematical explanation: $G_{i} \sum_{i=1}^{n} F_{t} \neq 0$ $\tau=\tau_{1}+\tau_{2}+\ldots...
A uniform sphere of mass m and radius R is placed on a rough horizontal surface. The sphere is struck horizontally at a height h from the floor.
Match the following $\begin{array}{|l|l|} \hline \text { a) } \mathrm{h}=\mathbf{R} / 2 & \text { i) sphere rolls without slipping with a constant velocity and no loss of energy } \\ \hline...
The variation of angular position θ, of a point on a rotating rigid body, with time t is shown in figure. Is the body rotating clock-wise or anti-clockwise?
We discover that the slope of the $\theta - t $ graph is positive, indicating anticlockwise rotation by convention. As $\theta$ is positive and we also know $\omega = \frac{d\theta}{dt}$ and $tan...
The centre of gravity of a body on the earth coincides with its centre of mass for a ‘small’ object whereas for an ‘extended’ object it may not. What is the qualitative meaning of ‘small’ and ‘extended’ in this regard? For which of the following the two coincides? A building, a pond, a lake, a mountain?
The geometric centre of gravity is the geometric centre, but the mass of the place where the complete mass of the body is considered is the mass of the centre of mass. When an object's vertical...
The net external torque on a system of particle about any axis is zero. Which of the following are compatible with it?
a) the forces may be acting radially from a point on the axis
b) the forces may be acting on the axis of rotation
c) the forces may be acting parallel to the axis of rotation
d) the torque caused by some forces may be equal and opposite to that caused by other forces
When net external torque on a system of particles about an axis is zero, torque is the cross prodeuct of $\vec r$ and $\vec F = rFsin\theta \times torque=0$ where, $\theta$ is the amgle between the...
Figure shows two identical particles 1 and 2, each of mass m, moving in opposite directions with the same speed v along parallel lines. At a particular instant, 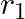 and
and 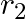 are their respective positions vectors drawn from point A which is in the plane of the parallel lines. Choose the correct options:
are their respective positions vectors drawn from point A which is in the plane of the parallel lines. Choose the correct options:
a) angular momentum 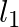 of particle 1 is about A is
of particle 1 is about A is 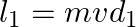
b) angular momentum 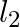 of particle 2 about A is
of particle 2 about A is 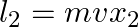
c) total angular momentum of the system about A is 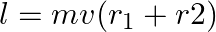
d) total angular momentum of the system about A is 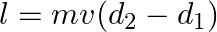
Solution: Correct answers is: d) total angular momentum of the system about A is $l = mv(d_{2}-d_{1})$ Angular momentum of particle 1 about A is given as, $\vec L_1=mvd_1$ Angular momentum of...
What are electron lacking mixtures? Are BCl3 and SiCl4 electron insufficient species? Clarify.
solution: Electron-lacking mixtures are substance compounds with inadequate octets that will in general get at least 1 electrons to finish their octet setups. BCl3 BCl3 is a genuine illustration of...
How might you clarify the higher soundness of BCl3 when contrasted with TlCl3?
Solution: Thallium and boron have a place with bunch 13 of the intermittent table and +1 oxidation state turns out to be more steady as we drop down the gathering. Boron is more steady than thallium...
Which one of the basic earth metal carbonates is thermally the most steady? (a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3
Solution: (d) BaCO3 Warm dependability is straightforwardly corresponding to the size of the cation i.e., bigger the size of the iota, more prominent is its warm security. The greatest cation among...
Which one of the accompanying soluble base metals gives hydrated salts? (a) Li (b) Na (c) K (d) Cs
Solution: (a) Li Li is fit for shaping hydrated salts in light of its size. Since it is more modest in size, it has a higher charge thickness and can undoubtedly draw in water atoms around it and...
Which of the accompanying soluble base metals has the most un-dissolving point? (a) Na (b) K (c) Rb (d) Cs
Solution: (d) Cs Cs has the most un-liquefying point of the given salt metals since it has the biggest size. Because of a bigger size, the limiting capacity of Cs is restricted and the grid energy...
How might you clarify the accompanying perceptions? (I) BeO is practically insoluble however BeSO4 is insoluble in water. (ii) BaO is solvent yet BaSO4 is insoluble in water. (iii) LiI is more solvent than KI in ethanol.
Solution: (I) The measures of Be2+ and O2-are little and are profoundly viable with one another. Because of this, a high measure of cross section energy is delivered during its arrangement. The...
State with regards to why (a) an answer of Na2CO3 basic in nature? (b) salt metals are ready by electrolysis of their combined chlorides? (c) Sodium is observed to be more helpful than potassium?
Solution: (a) Sodium bicarbonate and sodium hydroxide are the finished results when Na2CO3 is hydrolyzed. Since, the item are basic in nature, an answer of Na2CO3 is viewed as basic in nature. (b)...
Remark on every one of the accompanying perceptions: (a) The mobilities of the salt metal particles in fluid arrangement are Li+ < Na+ < K+ < Rb+ < Cs+ (b) Lithium is the main metal to frame a nitride straightforwardly. (c) ![Rendered by QuickLaTeX.com \[E0\text{ }for\text{ }M2+\left( aq \right)\text{ }+\text{ }2e\to \text{ }M\left( s \right)\text{ }\left( where\text{ }M\text{ }=\text{ }Ca,\text{ }Sr\text{ }or\text{ }Ba \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-30816136accb3333fde729011cce90bd_l3.png)
is almost steady.
Solution: (a) The ionic and nuclear sizes of the metals will in general increment while going down the antacid gathering. The expanding request of the ionic sizes of the salt metal particles is as...
Clarify the meaning of sodium, potassium, magnesium and calcium in organic liquids.
Solution: Sodium (Na): They are found in our blood plasma and the interstitial liquids around the cells. They help in (a) Transmission of nerve signals. (b) They direct the progression of water...
For what reason is LiF practically insoluble in water while LiCl dissolvable in water as well as in CH3)2CO?
Solution: LiF has a more noteworthy ionic person than LiCl which upsets the harmony between hydration energy and cross section energy. This equilibrium is critical for the reasonability of particles...
For what reason are lithium salts normally hydrated and those of the other antacid particles typically anhydrous?
Solution: Since Lithium has the littlest size among all the soluble base metals, it can without much of a stretch enraptured water atoms. Subsequently, more modest the size of the particle, more...
Portray the significance of the accompanying : (I) limestone (ii) concrete (iii) mortar of paris.
Solution: Employments of concrete: Extension development Putting Most significant fixing in concrete Employments of Plaster of Paris: Used to make projects and shape Used to make careful wraps...
The hydroxides and carbonates of sodium and potassium are effectively solvent in water while the relating salts of magnesium and calcium are sparingly dissolvable in water. Clarify.
solution: Since the nuclear sizes of magnesium and calcium are more modest than that of sodium and potassium, calcium and magnesium structure carbonates and hydroxides with higher cross section...
Draw the design of (I) BeCl2 (fume) (ii) BeCl2 (strong).
solution: BeCl2 has a straight construction and exists as a monomer in the fume state. 2. In the strong stage, BeCl2 is a polymer.
Depict two significant employments of every one of the accompanying : (I) harsh pop (ii) sodium carbonate (iii) quicklime.
solution: (I) Caustic pop (a) Heavily utilized in cleanser ventures. (b) Common reagent in research centers. (ii) Sodium carbonate (a) Finds utilizes in both cleanser and glass ventures. (b) Also...
For what reason is Li2CO3 deteriorated at a lower temperature while Na2CO3 at higher temperature?
Solution: The electropositive person increments while dropping down in the gathering of soluble base metal which brings about an increment in solidness of antacid carbonates. For the most part,...
Potassium carbonate can’t be ready by Solvay measure. Clarify why?
Solution: Solvay measure isn't pertinent for the arrangement of potassium carbonate since potassium carbonate is dissolvable in water and it doesn't hasten out like sodium bicarbonate.
Beryllium and magnesium don’t offer tone to fire though other basic earth metals do as such. Why?
solution: The valence electrons get eager to a higher energy level when a basic earth metal is warmed. It transmits energy which has a place with the apparent locale when this invigorated electron...
At the point when a soluble base metal breaks up in fluid alkali the arrangement can procure various tones. Clarify the explanations behind this kind of shading change.
solution: At the point when the soluble base metal is broken up in fluid alkali, a dark blue shaded arrangement is framed. \[M+\left( x+y \right)NH3\to M\left( NH3 \right)x+e-{}^\text{1}\text{...
A merry-go-round, made of a ring-like platform of radius R and mass M, is revolving with angular speed ω. A person of mass M is standing on it. At one instant, the person jumps off the ground, radially away from the centre of the round. The speed of the round afterwards is
a) 2ω
b) ω
c) ω/2
d) 0
Correct answer is a) 2ω
For what reason are potassium and caesium, as opposed to lithium utilized in photoelectric cells?
Solution: Lithium, potassium, and cesium, are all antacid metals. Yet at the same time, potassium and cesium are utilized in photoelectric cell and not Lithium since Li is more modest in size when...
The density of a non-uniform rod of length 1 m is given by 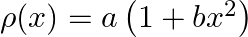 where a and b are constant and 0 ≤ x ≤ 1. The centre of mass of the rod will be at
where a and b are constant and 0 ≤ x ≤ 1. The centre of mass of the rod will be at
a) 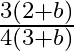
b) 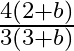
c) 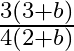
d) 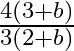
Correct answer is a) $\frac{3(2+b)}{4(3+b)}$ Considering a differential part of the rod at a distance of $x$, we have, $\begin{array}{l} l=\frac{d m}{d x}=a\left(1+b x^{2}\right) \Rightarrow d...
A uniform square plate has a small piece Q of an irregular shape removed and glued to the centre of the plate leaving a hole behind, the CM of the plate is now in the following quadrant of the x-y plane
a) I
b) II
c) III
d) IV
Correct answer is c) III
When a disc rotates with uniform angular velocity, which of the following is not true?
a) the sense of rotation remains the same
b) the orientation of the axis of rotation remains the same
c) the speed of rotation is non-zero and remains the same
d) the angular acceleration is non-zero and remains the same
Correct answer is d) the angular acceleration is non-zero and remains same.
A particle of mass m is moving in yz-plane with a uniform velocity v with its trajectory running parallel to +ve y-axis and intersecting z-axis at z = a. The change in its angular momentum about the origin as it bounces elastically from a wall at y = constant is
a) 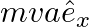
b) 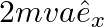
c) 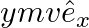
d) 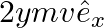
Solution: Correct answer is b) $2 m v a \hat{e}_{x}$
Which of the following points is the likely position of the centre of mass of the system shown in the figure
a) A
b) B
c) C
d) D
Solution: Correct answer is c) C
Clarify for what reason can antacid and soluble earth metals not be gotten by synthetic decrease strategies.
Solution: By utilizing a more grounded lessening specialist, the oxides of metals get diminished by the cycle called synthetic decrease. Basic earth metals and soluble base metals are solid among...
Analyze the salt metals and basic earth metals as for (I) ionization enthalpy (ii) basicity of oxides and (iii) solvency of hydroxides.
Solution: Alkaline earth metals Solubility of hydroxide: They are less soluble compared to alkali metals as it has high lattice energy and is having higher charge densities...
Clarify for what reason is sodium less responsive than potassium.
solution: On dropping down the gathering in the antacid metals, the size of the molecule increments and the impact of the atomic charge gets diminished. Because of these elements, the electron of...
Discover the oxidation condition of sodium in Na2O2.
Solution: Leave the oxidation territory of Na alone y. In the event of peroxides, the oxidation condition of oxygen is - 1. Subsequently, \[\begin{array}{*{35}{l}} ~ \\ 2\left( y...
For what reason are antacid metals not found in nature?
Solution: Sodium, cesium, lithium, francium, potassium, rubidium all together include the salt metals. They comprise of just a single electron on its valence shell, which gets free effectively...
Examine the overall qualities and degree in properties of basic earth metals
Solution: General attributes: (I) (Noble gas) ns2 is the electronic design of basic earth metal. (ii) To possess the closest inactive gas setup, these metals lose two of their...
What are the normal physical and synthetic provisions of soluble base metals?
Solution: Actual properties: (1) The soluble base metal is delicate thus we can cut them without any problem. We can ready to cut the sodium metal even by utilizing the blade. (2)...
The enthalpies of all components in their standard states are: (I) Unity (ii) Zero (iii) < 0 (iv) Different for each component
solution: (ii) Zero
For the interaction to happen under adiabatic conditions, the right condition is: ![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }T\text{ }=\text{ }0 \\ ~\left( ii \right)\text{ }p\text{ }=\text{ }0 \\ \left( iii \right)\text{ }q\text{ }=\text{ }0 \\ ~\left( iv \right)\text{ }w\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-68d24071d9398f3df1fc5ee9b1897869_l3.png)
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} \left( I \right)\text{ }T\text{ }=\text{ }0 \\ ~\left( ii \right)\text{ }p\text{ }=\text{ }0 \\ \left( iii \right)\text{ }q\text{ }=\text{ }0 \\ ~\left( iv \right)\text{ }w\text{ }=\text{ }0 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-68d24071d9398f3df1fc5ee9b1897869_l3.png)
solution: \[\left( iii \right)\text{ }q\text{ }=\text{ }0\] Reason: For an adiabatic interaction heat move is zero, for example \[q\text{ }=\text{ }0.\]
Pick the right reply. A thermodynamic state work is an amount (I) used to decide heat changes (ii) whose worth is free of way (iii) used to decide pressure volume work (iv) whose worth relies upon temperature as it were
(ii) An amount which is autonomous of way. solution: Capacities like strain, volume and temperature relies upon the condition of the framework just and not on the way.
Clarify the actual meaning of Van der Waals boundaries?
solution: The actual meaning of 'a': The greatness of intermolecular appealing powers inside gas is addressed by 'a'. The actual meaning of 'b': The volume of a gas atom is addressed by...
Basic temperature for carbon dioxide and methane are 31.1 °C and – 81.9 °C individually. Which of these has more grounded intermolecular powers and why?
solution: In the event that the basic temperature of a gas is higher, it is simpler to condense. That is the intermolecular powers of fascination among the atoms of gas are straightforwardly...
As far as Charles’ law clarify why – 273°C is the most minimal conceivable temperature.
solution: Charles saw that the volume of certain measure of a gas changes by VO /273.15 for every degree rise or fall in temperature.VO being the volume at 0°C.The volume at any temperature t°C...
What might be the SI unit for the amount pV2T 2/n?
solution: The SI unit for pressure, p is Nm–2 . The SI unit for volume, V is m3. The SI unit for temperature, T is K. The SI unit for the quantity of moles, n is mol. Subsequently, the SI unit for...
A combination of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Compute the halfway tension of dihydrogen.
solution: Let the heaviness of dihydrogen be 20 g. Let the heaviness of dioxygen be 80 g. No. of moles of dihydrogen (nH2), \[\begin{array}{*{35}{l}} ~ \\ =\text{ }20/2 \\ ~ \\...
Work out the volume involved by 8.8 g of CO2 at 31.1°C and 1 bar pressure. ![Rendered by QuickLaTeX.com \[R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-63d0fbfbaf1b21bdbcb7644d8def9148_l3.png)
solution: \[\begin{array}{*{35}{l}} ~ \\ pVM\text{ }=\text{ }mRT \\ ~ \\ V=\text{ }mRT/Mp \\ \end{array}\] Here, \[\begin{array}{*{35}{l}} m\text{ }=\text{ }8.8\text{ }g \\ ~ \\ R\text{...
Payload is characterized as the contrast between the mass of uprooted air and the mass of the inflatable. Work out the payload when an inflatable of sweep 10 m, mass 100 kg is loaded up with helium at 1.66 bar at 27°C. ![Rendered by QuickLaTeX.com \[~\left( Thickness\text{ }of\text{ }air\text{ }=\text{ }1.2\text{ }kg\text{ }m3\text{ }and\text{ }R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1 \right)\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-106263d412dec0c1dbf32f23a40c938c_l3.png)
solution: Given: \[r\text{ }=\text{ }10\text{ }m\] Accordingly, volume of the inflatable \[\begin{array}{*{35}{l}} =4/3\text{ }\pi r{}^\text{3} \\ ~ \\ =4/3\text{ }\times...
Work out the all out strain in a combination of 8 g of dioxygen and 4 g of dihydrogen restricted in a vessel of 1 dm3 at 27°C. ![Rendered by QuickLaTeX.com \[R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-48f6d7112751bbb16471c7a7ca51ec8d_l3.png)
solution : Given: \[Mass\text{ }of\text{ }O2\text{ }=\text{ }8\text{ }g\]
What amount of time would it require to circulate one Avogadro number of wheat grains, if 1010 grains are appropriated each second?
solution: \[Avogadro\text{ }number\text{ }=\text{ }6.02\text{ }\times \text{ }1023\]Thus, time required
Ascertain the complete number of electrons present in 1.4 g of dinitrogen gas.
solution: \[Molar\text{ }mass\text{ }of\text{ }dinitrogen\text{ }\left( N2 \right)\text{ }=\text{ }28\text{ }g\text{ }mol-1\] Subsequently, 1.4 g of N2 \[\begin{array}{*{35}{l}} 1.4/2.8 \\ ~ \\...
Work out the temperature of 4.0 mol of a gas involving 5 dm3 at 3.32 bar. ![Rendered by QuickLaTeX.com \[\left( R\text{ }=\text{ }0.083\text{ }bar\text{ }dm3\text{ }K1\text{ }mol1 \right).\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0eea6131e45ed685db113cdc3bf5955a_l3.png)
Solution: Given, \[\begin{array}{*{35}{l}} Measure\text{ }of\text{ }the\text{ }gas\text{ },n~=\text{ }4.0\text{ }mol \\ ~ \\ Volume\text{ }of\text{ }the\text{ }gas~,V~=\text{ }5\text{ }dm3 ...
An understudy neglected to add the response blend to the round lined cup at 27 °C however rather he/she set the flagon on the fire. After a pass of time, he understood his error, and utilizing a pyrometer he discovered the temperature of the carafe was 477 °C. What part of air would have been removed out?
solution: Leave the volume of the round lined cup alone V. Then, at that point, the volume of air inside the cup at 27° C is V. Presently, \[\begin{array}{*{35}{l}} ~ \\ V1\text{...
34.05 mL of phosphorus fume weighs 0.0625 g at 546 °C and 0.1 bar pressure. What is the molar mass of phosphorus?
solution: Given, \[\begin{array}{*{35}{l}} p\text{ }=\text{ }0.1\text{ }bar \\ ~ \\ V\text{ }=\text{ }34.05\text{ }mL\text{ }=\text{ }34.05\text{ }\times \text{ }103\text{ }L\text{ }=\text{...
The thickness of a gas is observed to be 5.46 g/dm3 at 27 °C at 2 bar pressure. What will be its thickness at STP?
Solution: For an optimal gas \[Thickness\text{ }\rho \text{ }=\text{ }p\text{ }x\text{ }M/RT\] From the given information, \[\begin{array}{*{35}{l}} 5.46\text{ }=\text{ }2\text{...
What will be the tension of the vaporous blend when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are presented in a 1L vessel at 27°C?
Solution: From the situation \[Pv\text{ }=\text{ }n\text{ }RT\] for the two gases. We can compose \[\begin{array}{*{35}{l}} 0.8\text{ }x\text{ }0.5\text{ }=\text{ }nH2~x\text{ }RT~\text{...
In an experiment to estimate the size of a molecule of oleic acid, 1mL of oleic acid is dissolved in 19mL of alcohol. Then 1mL of this solution is diluted to 20mL by adding alcohol. Now, 1 drop of this diluted solution is placed on water in a shallow trough. The solution spreads over the surface of water forming one molecule thick layer. Now, lycopodium powder is sprinkled evenly over the film we can calculate the thickness of the film which will give us the size of oleic acid molecule.
Read the passage carefully and answer the following questions:
a) Why do we dissolve oleic acid in alcohol?
b) What is the role of lycopodium powder?
a) Because oleic acid does not dissolve in water, it is dissolved in alcohol. b) When oleic acid is introduced, lycopodium powder clears the circular area. This makes it possible to measure the area...
An artificial satellite is revolving around a planet of mass M and radius R, in a circular orbit of radius r. From Kepler’s third law about the period of a satellite around a common central body, square of the period of revolution T is proportional to the cube of the radius of the orbit r. Show using dimensional analysis, that T = k/R √r3/g where k is a dimensionless constant and g is acceleration due to gravity.
Kepler's third law states that, $T^{2} \propto a^{3}$ i.e., square of time period $\left(T^{2}\right)$ of a satellite revolving around a planet, is proportional to the cube of the radius of the...
The channel cleaner, Drainex contains little pieces of aluminum which respond with scathing soft drink to create dihydrogen. What volume of dihydrogen at 20 °C and one bar will be delivered when 0.15g of aluminum responds?
solution: Aluminum responds with scathing soft drink as per the response The response of aluminum with scathing soft drink can be addressed as: \[2Al\text{ }+\text{ }2NaOH\text{...
The tension of 1 g of an ideal gas An at 27 °C is observed to be 2 bar. At the point when 2 g of one more ideal gas B is presented in a similar flagon at a similar temperature the strain becomes 3 bar. Discover a connection between their sub-atomic masses.
Solution: \[\begin{array}{*{35}{l}} Mass\text{ }of\text{ }gas\text{ }A\text{ },\text{ }WA~=\text{ }1g \\ ~ \\ Mass\text{ }of\text{ }gas\text{ }B,~\text{ }WB~=\text{ }2g \\ ~ \\ Tension\text{...
At 0°C, the thickness of a specific oxide of a gas at 2 bar is equivalent to that of dinitrogen at 5 bar. What is the sub-atomic mass of the oxide?
Utilizing the relationship of thickness (ρ) of the substance at temperature (T) We have \[\rho \text{ }=\text{ }Mp/RT\] or on the other hand \[p\text{ }=\text{ }\rho \text{ }RT/M\]...
In the expression 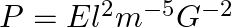 , E, m, l and G denote energy, mass, angular momentum and gravitational constant, respectively. Show that P is a dimensionless quantity.
, E, m, l and G denote energy, mass, angular momentum and gravitational constant, respectively. Show that P is a dimensionless quantity.
We have, $P=El^{2}m^{-5}G^{-2}$ E is the energy having dimension $ML^{2}T{-2}$ m is the mass having dimension [M] L is the angular momentum having dimension $[ML^{2}T^{1}]$ G is the gravitational...
A vessel of 120 mL limit contains a specific measure of gas at 35 °C and 1.2 bar pressure. The gas is moved to one more vessel of volume 180 mL at 35 °C. What might be its strain?
As indicated by Boyle's Law \[P1~V1~=\text{ }P2~V2\] Here the temperature is steady. Thusly \[1.2\text{ }X\text{ }120\text{ }=\text{ }P2~X\text{ }180\] Or then again...
What will be the base strain needed to pack 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Volume of gas\[\left( V1 \right)\text{ }=\text{ }500dm3~~\] (given) Strain of gas \[\left( p1 \right)\text{ }=\text{ }1\text{ }bar\text{ }~~\] (given) Volume of packed gas \[\left( V2...
The volume of a liquid flowing out per second of a pipe of length l and radius r is written by a student as 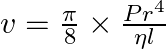 where P is the pressure difference between the two ends of the pipe and η is coefficient of viscosity of the liquid having dimensional formula
where P is the pressure difference between the two ends of the pipe and η is coefficient of viscosity of the liquid having dimensional formula 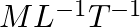 . Check whether the equation is dimensionally correct.
. Check whether the equation is dimensionally correct.
Dimension of the given physical quantity is as follows, [V] = dimension of volume/dimension of time $=[L^{3}]/[T]$ $=[M^{-1}T^{-2}]$ LHS $=[L^{3}T^{-1}]$ RHS $=[L^{3}T^{-1}]$ LHS = RHS Hence, the...
The vernier scale of a travelling microscope has 50 divisions which coincide with 49 main scale divisions. If each main scale division is 0.5 mm, calculate the minimum inaccuracy in the measurement of distance.
The minimum inaccuracy in the measurement of distance = (1/50)(0/5)mm = 0.01 mm
The distance of a galaxy is of the order of 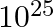 m. Calculate the order of magnitude of time taken by light to reach us from the galaxy.
m. Calculate the order of magnitude of time taken by light to reach us from the galaxy.
The distance of the galaxy is given as 1025m Speed of light is known as $3\times 10^{8}m/s$ Time taken is t = distance/speed $=3.33\times 10^{16}s$
Which of the following time measuring devices is most precise?
(a) A wall clock.
(b) A stopwatch.
(c) A digital watch.
(d) An atomic clock.
Give the reason for your answer.
Correct option is d) an atomic clock as it measures up to one second.
(a) The earth-moon distance is about 60 earth radius. What will be the diameter of the earth (approximately in degrees) as seen from the moon?
(b) Moon is seen to be of (½)° diameter from the earth. What must be the relative size compared to the earth?
(a) Because the distance between the moon and the earth is greater than the radius of the earth, it is considered as an arc. Let the length of the arc be $R_{e}$ Distance between the moon and the...
Why do we have different units for the same physical quantity?
Because physical quantities differ from location to place, we have several units for the same physical quantity.
If Planck’s constant (h) and speed of light in vacuum (c) are taken as two fundamental quantities, which of the following can, also, be taken to express length, mass, and time in terms of the three chosen fundamental quantities?
a) mass of the electron 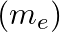
b) universal gravitational constant 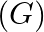
c) charge of the electron 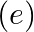
d) mass of proton 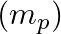
Correct answers are a) mass of electron b) universal gravitational constant and d) mass of proton
Young’s modulus of steel is 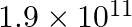
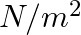 . When expressed in CGS units of
. When expressed in CGS units of 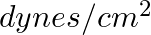 , it will be equal to:
, it will be equal to:
a) 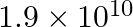
b) 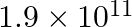
c) 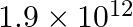
d) 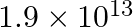
Correct answer is c) $1.9\times 10^{12}$ Both Dyne and Newton are force units. While Dyne is measured in the C-G-S (Centimeter – Gram – Second) system, Newton is measured in the contemporary SI...
Measure of two quantities along with the precision of the respective measuring instrument is:
A = 2.5 m/s ± 0.5 m/s
B = 0.10 s ± 0.01 s
The value of AB will be
a) (0.25 ± 0.08) m
b) (0.25 ± 0.5) m
c) (0.25 ± 0.05) m
d) (0.25 ± 0.135) m
Correct answer is a) (0.25 ± 0.08) m Here, $\mathrm{A}=2.5 \mathrm{~ms}^{-1} \pm 0.5 \mathrm{~ms}^{-1}, \mathrm{~B}=0.10 \mathrm{~s} \pm 0.01 \mathrm{~s}$ $\mathrm{AB}=\left(2.5...
Which of the following pairs of physical quantities does not have the same dimensional formula?
a) work and torque
b) angular momentum and Planck’s constant
c) tension and surface tension
d) impulse and linear momentum.
Correct answer is c) Tension and surface tension. Tension has the dimension: $[MLT^-2]$ Surface Tension has the dimension: $[ML^0T^-2]$
The length and breadth of a rectangular sheet are 16.2 cm and 10.1 cm respectively. The area of the sheet inappropriate significant figures and error is:
a) 164 ± 3 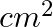
b) 163.62 ± 2.6 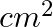
c) 163.6 ± 2.6 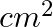
d) 163.62 ± 3 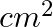
Correct answer is a) 164 ± 3 $cm^{2}$ Error in product of quantities: Suppose $x=a \times b$ Let, $\Delta a$ be the absolute error in measurement of a, $\Delta b$ be the absolute error in...
The numbers 2.745 and 2.735 on rounding off to 3 significant figures will give:
a) 2.75 and 2.74
b) 2.74 and 2.73
c) 2.75 and 2.73
d) 2.74 and 2.74
Correct answer is d) 2.74 and 2.74 By convention, we apply the following criteria for rounding off measurements: 1. If the dropped digit is less than 5, the preceding digit remains unaffected. 2. If...
The sum of the numbers 436.32, 227.2, and 0.301 inappropriate significant figures is:
a) 663.821
b) 664
c) 663.8
d) 663.82
Correct answer is c) 663.8
Which of the following statement is not true about amorphous solids?
(i) On heating, they may become crystalline at a certain temperature. (ii) They may become crystalline on keeping for a long time. (iii) Amorphous solids can be moulded by heating. (iv) They are...
Which of the following is true about the value of the refractive index of quartz glass?
(i) Same in all directions (ii) Different in different directions (iii) Cannot be measured (iv) Always zero Correct Answer: (i) Same in all directions Explanation: As the amorphous...
Which of the following arrangements shows the schematic alignment of magnetic moments of antiferromagnetic substances?
Correct Answer: Option (d) Explanation: These are the factors in which the electron spins are associated with magnetic atoms in certain crystallographic areas and are directed at each other in such...
Which of the following is an amorphous solid?
(i) Graphite (C) (ii) Quartz glass (SiO2) (iii) Chrome alum (iv) Silicon carbide (SiC) Correct Answer: (ii) Quartz glass (SiO2) Explanation: Solids are considered as an amorphous when they...
Which of the following is not a characteristic of a crystalline solid?
(i) Definite and characteristic heat of fusion (ii) Isotropic nature (iii) A regular periodically repeated pattern of arrangement of constituent particles in the entire crystal (iv) A true solid...
Which of the following conditions favours the existence of a substance in the solid state?
(i) High temperature (ii) Low temperature (iii) High thermal energy (iv) Weak cohesive forces Correct Answer: (ii) Low temperature Explanation: At the low temperatures, the substances...
Pressure versus volume graph for a real gas and an ideal gas is shown in Fig. 5.4. Answer the following questions based on this graph. (i) Interpret the behaviour of real gas with respect to an ideal gas at low pressure. (ii) Interpret the behaviour of real gas with respect to an ideal gas at high pressure. (iii)Mark the pressure and volume by drawing a line at the point where real gas behaves as an ideal gas.
(i) At low pressure as the dark blue curve and the sky blue curve are approaching each other, it shows that the real gas is behaving as an ideal gas at a low pressure. (ii) At high pressure as the...
The variation of pressure with the volume of the gas at different temperatures can be graphically represented as shown in Fig. 5.3. Based on this graph answer the following questions. (i) How will the volume of a gas change if its pressure is increased at constant temperature? (ii) At constant pressure, how will the volume of a gas change if the temperature is increased from 200K to 400K?
(i) As the temperature is constant, and the pressure is increasing and the change in the volume is seen as exponentially decreasing. (ii) At constant pressure, by increasing the temperature there is...
Explain the effect of increasing the temperature of a liquid, on intermolecular forces operating between its particles, what will happen to the viscosity of a liquid if its temperature is increased?
As the temperature increases, the intermolecular force operating between the particles decreases, the bond strength increases and also the kinetic energy increases. Hence, as the temperature...
The viscosity of a liquid arises due to strong intermolecular forces existing between the molecules. Stronger the intermolecular forces, greater is the viscosity. Name the intermolecular forces existing in the following liquids and arrange them in the increasing order of their viscosities. Also, give a reason for the assigned order in one line. Water, hexane (CH3CH2CH2CH2CH2CH3), glycerine (CH2 OH CH(OH) CH2 OH)
Water has hydrogen bonding that exists as intermolecular force of attraction, hexane has Vander Waal force of attraction existing as intermolecular force of attraction, glycerin also has hydrogen...
A mass attached to a spring is free to oscillate, with angular velocity 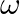 , in a horizontal plane without friction or damping. It is pulled to a distance
, in a horizontal plane without friction or damping. It is pulled to a distance 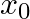 and pushed towards the centre with a velocity
and pushed towards the centre with a velocity 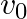 at time
at time 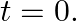 Determine the amplitude of the resulting oscillations in terms of the parameters
Determine the amplitude of the resulting oscillations in terms of the parameters 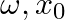 and
and 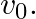 [Hint: Start with the equation
[Hint: Start with the equation 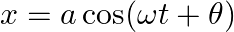 and note that the initial velocity is negative.]
and note that the initial velocity is negative.]
The angular velocity of the spring be $\omega$ $x=a \cos (\omega t+\theta)$ At $t=0, x=x_{0}$ On Substituting these values in the above equation we get, $\mathrm{x}_{0}=\mathrm{A} \cos \theta-(1)$...
Name two phenomena that can be explained on the basis of surface tension.
Bubbles have a round surface due to surface tension. A needle can float in water because of the presence of surface tension on the surface of the water.
A body describes simple harmonic motion with an amplitude of 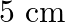 and a period of
and a period of 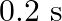 . Find the acceleration and velocity of the body when the displacement is 0
. Find the acceleration and velocity of the body when the displacement is 0 
Amplitude is given as $=5 \mathrm{~cm}=0.05 \mathrm{~m}$ Time period is given as $=0.2 \mathrm{~s}$ When the displacement is $y$, then acceleration is given as $A=-\omega^{2} y$ Velocity is given as...
The relation between the pressure exerted by an ideal gas (Pideal) and observed pressure (Pearl) is given by the equation: Pideal = Preal+ an2/V2 If the pressure is taken in Nm-2, the number of moles in mol and volume in m3, Calculate the unit of ‘a’. What will be the unit of ‘a’ when pressure is in atmosphere and volume in dm3?
We know that: Pideal = Preal + an2/V2 Pideal – Preal= an2/V2 Nm-2 = a*mol2/m6 A = Nm4mol-2 The unit of ‘a’ when the pressure is taken in Nm-2, number of moles in “mol” and volume in m3 is Nm4mol-2...
A body describes simple harmonic motion with an amplitude of 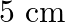 and a period of
and a period of 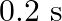 . Find the acceleration and velocity of the body when the displacement is
. Find the acceleration and velocity of the body when the displacement is
(a) 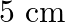
(b) 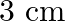
Amplitude is given as $=5 \mathrm{~cm}=0.05 \mathrm{~m}$ Time period is given as $=0.2 \mathrm{~s}$ When the displacement is $y$, then acceleration is given as $A=-\omega^{2} y$ Velocity is given as...
For real gases the relation between p, V and T are given by van der Waals equation: [(P + an2) / V2](V – nb) = nRT Where‘a’ and ‘b’ are van der Waals constants, ‘nb’ is approximately equal to the total volume of the molecules of a gas. ‘a’ is the measure of the magnitude of intermolecular attraction. (i) Arrange the following gases in the increasing order of ‘b’. Give reason. O2, CO2, H2, He (ii) Arrange the following gases in the decreasing order of magnitude of ‘a’. Give reason. CH4, O2, H2
(i) The increasing order of ‘b’ is as follows: He < H2< O2< CO2. As the Vander Waals constant ‘b’ is approximately equal to the total volume of the molecules of a gas. (ii)The decreasing...
A circular disc of mass 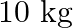 is suspended by a wire attached to its centre. The wire is twisted by rotating the disc and released. The period of torsional oscillations is found to be
is suspended by a wire attached to its centre. The wire is twisted by rotating the disc and released. The period of torsional oscillations is found to be 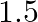 s. The radius of the disc is
s. The radius of the disc is 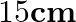 . Determine the torsional spring constant of the wire. (Torsional spring constant
. Determine the torsional spring constant of the wire. (Torsional spring constant  is defined by the relation
is defined by the relation 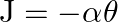 , where
, where 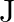 is the restoring couple and
is the restoring couple and 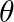 the angle of twist).
the angle of twist).
Mass of the circular disc is given as $10 \mathrm{~kg}$ Period of torsional oscillation is given as $1.5 \mathrm{~s}$ Radius of the disc is given as $15 \mathrm{~cm}=0.15 \mathrm{~m}$ Restoring...
You are riding in an automobile of mass 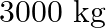 . Assuming that you are examining the oscillation characteristics of its suspension system. The suspension sags
. Assuming that you are examining the oscillation characteristics of its suspension system. The suspension sags 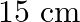 when the entire automobile is placed on it. Also, the amplitude of oscillation decreases by
when the entire automobile is placed on it. Also, the amplitude of oscillation decreases by 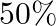 during one complete oscillation. Estimate the values of (a) the spring constant
during one complete oscillation. Estimate the values of (a) the spring constant 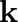 and (b) the damping constant b for the spring and shock absorber system of one wheel, assuming that each wheel supports
and (b) the damping constant b for the spring and shock absorber system of one wheel, assuming that each wheel supports  .
.
(a) Mass of the automobile is given as $=3000 \mathrm{~kg}$ The suspension sags by a length of $15 \mathrm{~cm}$ Decrease in amplitude $=50 \%$ during one complete oscillation If each spring's...
One end of a U-tube containing mercury is connected to a suction pump and the other end to atmosphere. A small pressure difference is maintained between the two columns. Show that, when the suction pump is removed, the column of mercury in the U-tube executes simple harmonic motion.
Area of cross-section of the U-tube is given as $A$ Density of the mercury column is given as $\rho$ Acceleration due to gravity is given as $g$ Restoring force, F = Weight of the mercury column of...
A simple pendulum of length I and having a bob of mass 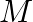 is suspended in a car. The car is moving on a circular track of radius
is suspended in a car. The car is moving on a circular track of radius 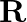 with a uniform speed
with a uniform speed 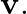 If the pendulum makes small oscillations in a radial direction about its equilibrium position, what will be its time period?
If the pendulum makes small oscillations in a radial direction about its equilibrium position, what will be its time period?
The centripetal acceleration supplied by the circular motion of the car, as well as the acceleration due to gravity, will be felt by the bob of the basic pendulum. Acceleration due to gravity is...
Answer the following questions:
(a) Time period of a particle in SHM depends on the force constant 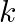 and mass
and mass 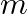 of the particle:
of the particle: 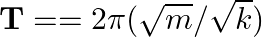 . A simple pendulum executes SHM approximately. Why then is the time period of a pendulum independent of the mass of the pendulum?
. A simple pendulum executes SHM approximately. Why then is the time period of a pendulum independent of the mass of the pendulum?
(b) The motion of a simple pendulum is approximately simple harmonic for small-angle oscillations. For larger angles of oscillation, a more involved analysis shows that  is greater than
is greater than 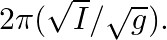 Think of a qualitative argument to appreciate this result.
Think of a qualitative argument to appreciate this result.
(a) The spring constant $k$ is proportional to the mass in the case of a simple pendulum. The numerator ($m$) and denominator ($d$) will cancel each other out. As a result, the simple pendulum's...
The acceleration due to gravity on the surface of moon is 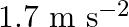 . What is the time period of a simple pendulum on the surface of moon if its time period on the surface of earth is
. What is the time period of a simple pendulum on the surface of moon if its time period on the surface of earth is 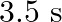 ? (g on the surface of earth is
? (g on the surface of earth is 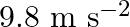 )
)
Acceleration due to gravity on the surface of moon is given as $g^{\prime}=1.7 \mathrm{~m} \mathrm{~s}^{-2}$ Acceleration due to gravity on the surface of earth is given as $g=9.8 \mathrm{~m}...
The piston in the cylinder head of a locomotive has a stroke (twice the amplitude) of 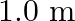 . If the piston moves with simple harmonic motion with an angular frequency of
. If the piston moves with simple harmonic motion with an angular frequency of 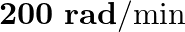 , what is its maximum speed?
, what is its maximum speed?
Angular frequency of the piston is given as $\omega=200 \mathrm{rad} / \mathrm{min}$ Stroke is given as$=1.0 \mathrm{~m}$ Amplitude is given as $A=1.0 / 2$ $=0.5 \mathrm{~m}$ The maximum speed...
Figure (a) shows a spring of force constant k clamped rigidly at one end and a mass 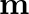 attached to its free end. A force
attached to its free end. A force 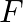 applied at the free end stretches the spring. Figure (b) shows the same spring with both ends free and attached to a mass
applied at the free end stretches the spring. Figure (b) shows the same spring with both ends free and attached to a mass 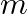 at either end. Each end of the spring in Fig. (b) is stretched by the same force F.
at either end. Each end of the spring in Fig. (b) is stretched by the same force F.
(a) What is the maximum extension of the spring in the two cases? (b) If the mass in Fig. (a) and the two masses in Fig. (b) is released, what is the period of oscillation in each case? Solution:...
Plot the corresponding reference circle for each of the following simple harmonic motions. Indicate the initial  position of the particle, the radius of the circle, and the angular speed of the rotating particle. For simplicity, the sense of rotation may be fixed to be anticlockwise in every case: (
position of the particle, the radius of the circle, and the angular speed of the rotating particle. For simplicity, the sense of rotation may be fixed to be anticlockwise in every case: ( 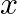 is in cm and
is in cm and 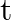 is in
is in 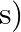 .
.
(a) 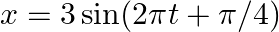 (b)
(b) 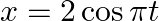
(a)$x=3 \sin (2 m t+\pi / 4)$ $=-3 \cos (2 \pi t+\pi / 4+\pi / 2)$ $=-3 \cos (2 \pi t+3 \pi / 4)$ $=-3 \cos (2 \pi t+3 \pi / 4)$ On comparing with the standard equation $A \cos (\omega t+\Phi)$, we...
Some time ago the formation of polar stratospheric clouds was reported over Antarctica. Why were these formed? What happens when such clouds break up by the warmth of sunlight?
The ozone layer is being depleted. During the summer, nitrogen dioxide and methane interacted with chlorine monoxide and chlorine radicle, respectively, to produce chlorine sinks, avoiding ozone...
The given figures correspond to two circular motions. The radius of the circle, the period of revolution, the initial position, and the sense of revolution (i.e. clockwise or anti-clockwise) are indicated on each figure.
Obtain the corresponding simple harmonic motions of the $x$-projection of the radius vector of the revolving particle $P$, in each case. Solution: (a) Time period is given as $t=2 \mathrm{~s}$...
In the given figure, let us take the position of mass when the spring is unstreched as 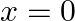 , and the direction from left to right as the positive direction of
, and the direction from left to right as the positive direction of 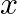 -axis. Give
-axis. Give 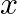 as a function of time
as a function of time 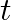 for the oscillating mass if at the moment we start the stopwatch (t
for the oscillating mass if at the moment we start the stopwatch (t 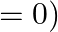 , the mass is
, the mass is
(a) at the mean position,
(b) at the maximum stretched position.
Solution: Distance travelled by the mass sideways is given as $a=2.0 \mathrm{~cm}$ Angular frequency of oscillation can be calculated as, $\omega=\sqrt{k} / \mathrm{m}$ $=\sqrt{1200 / 3}$...
A spring having with a spring constant 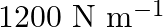 is mounted on a horizontal table as shown in Fig. 14.24. A mass of
is mounted on a horizontal table as shown in Fig. 14.24. A mass of 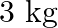 is attached to the free end of the spring. The mass is then pulled sideways to a distance of
is attached to the free end of the spring. The mass is then pulled sideways to a distance of 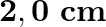 and released.
and released.
Determine<br>(i) the frequency of oscillations, (ii) maximum acceleration of the mass Solution: Spring constant is given as $\mathrm{k}=1200 \mathrm{~N} \mathrm{~m}^{-1}$ Mass is given as...
The motion of a particle executing simple harmonic motion is described by the displacement function, 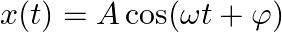 If the initial (t
If the initial (t  ) position of the particle is
) position of the particle is  and its initial velocity is
and its initial velocity is 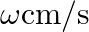 , what are its amplitude and initial phase angle? The angular frequency of the particle is
, what are its amplitude and initial phase angle? The angular frequency of the particle is 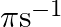 . If instead of the cosine function, we choose the sine function to describe the SHM:
. If instead of the cosine function, we choose the sine function to describe the SHM: 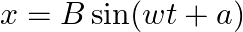 , what are the amplitude and initial phase of the particle with the above initial conditions. Solution:
, what are the amplitude and initial phase of the particle with the above initial conditions. Solution:
At positlon, t = 0, The given function is $x(t)=A \cos (\omega t+\phi).....(1)$ $\begin{array}{l} 1=A \cos (\omega \times 0+\phi)=A \cos \phi \\ A \cos \phi=1 \end{array}$ Differentiating equation...
Which of the following relationships between the acceleration a and the displacement 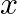 of a particle involve simple harmonic motion?
of a particle involve simple harmonic motion?
(a) 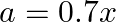
(b) 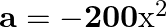
Condition of SHM is stated below, Acceleration is directly proportional to negative of displacement of particle. If ' $\mathrm{a}$ ' is the acceleration, $\mathrm{x}$ is the displacement Then, for...
A particle is in linear simple harmonic motion between two points, A and B, 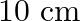 apart. Take the direction from
apart. Take the direction from  to
to  as the positive direction and give the signs of velocity, acceleration and force on the particle when it is
as the positive direction and give the signs of velocity, acceleration and force on the particle when it is
(a) at the mid-point of AB going towards A,
(b) at 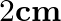 away from B going towards
away from B going towards 
(a) Negative, Zero, Zero A basic harmonic motion is being performed by the particle. The particle's mean location is denoted by $O$. Its highest velocity is at the mean position $O$. Because the...
A particle is in linear simple harmonic motion between two points, A and B, 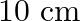 apart. Take the direction from
apart. Take the direction from  to
to 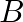 as the positive direction and give the signs of velocity, acceleration and force on the particle when it is
as the positive direction and give the signs of velocity, acceleration and force on the particle when it is
(a) at the end 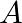 ,
,
(b) at the end B
(a) Zero, Positive, Positive Points A and B are the path's two ends, with A-B=10cm and'O' being the path's halfway. Between the end locations, a particle moves in a linear simple harmonic motion....
Which of the following functions of time represent (a) simple harmonic, (b) periodic but not simple harmonic, and (c) non-periodic motion? Give period for each case of periodic motion (w is any positive constant):
(a) 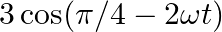
(b) 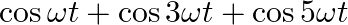
(a) $3 \cos [4 \pi-2 \omega t]=3 \cos [2 \omega t-\pi / 4]$ The equation can be written in the form $\cos (\omega t+\phi)$. It is S.H.M with the period $2 \pi / 2 \omega=\pi / \omega$ (b) $\cos...
Which of the following functions of time represent (a) simple harmonic, (b) periodic but not simple harmonic, and (c) non-periodic motion? Give period for each case of periodic motion (w is any positive constant):
(a) 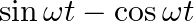
(b) 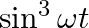
(a) $\sin \omega t-\cos \omega t$ =2[(1/2)sinωt-(1/2)cosωt] =\sqrt{2}[(1 / \sqrt{2}) \sin \omega t-(1 / \sqrt{2}) \cos \omega t] $=\sqrt{2}[\sin \omega t \times \cos (\pi / 4)-\cos...
The critical temperature (Tc) and critical pressure (Pc) of CO2 are 30.98°C and 73atm respectively. Can CO2(g) be liquefied at 32°C and 80atm pressure?
CO2 gas cannot be liquefied at a temperature which is greater than its critical temperature i.e 30.98°C even by applying any pressure. So as the given temperature is 32°C by applying a pressure of...
Compressibility factor, Z, of a gas is given as Z = PV/ nRT (i) What is the value of Z for an ideal gas? (ii) For real gas what will be the effect on the value of Z above Boyle’s temperature?
(i) Compressibility factor, Z is defined as the ratio of the product of pressure and volume to the product of the number of moles, gas constant and temperature. For an ideal gas, the value of Z is...
The figures depicts  plots for linear motion of a particle. Which of the plots represent periodic motion? What is the period of motion (in case of periodic motion)?
plots for linear motion of a particle. Which of the plots represent periodic motion? What is the period of motion (in case of periodic motion)?
(a) Because the motion is repeated in only one position, the depicted graph does not illustrate periodic motion. The full motion during one period must be repeated successively for a periodic...
The figures depicts  plots for linear motion of a particle. Which of the plots represent periodic motion? What is the period of motion (in case of periodic motion)?
plots for linear motion of a particle. Which of the plots represent periodic motion? What is the period of motion (in case of periodic motion)?
(a) Motion is not periodic since it does not repeat itself after a set length of time. (b) The following graph depicts a periodic motion that repeats every 2 seconds.
Which of the following examples represent (nearly) simple harmonic motion and which represent periodic but not simple harmonic motion?
(a) motion of a ball bearing inside a smooth curved bowl, when released from a point slightly above the lower most point.
(b) general vibrations of a polyatomic molecule about its equilibrium position.
(a) Simple harmonic motion (b) SHM is not periodic, although general vibrations of a polyatomic molecule about its equilibrium position are. The inherent frequencies of a polyatomic molecule are...
Which of the following examples represents periodic motion?
(a) A hydrogen molecule rotating about its centre of mass.
(b) An arrow released from a bow.
(a) The rotation of a hydrogen molecule around its mass centre is periodic. This is due to the fact that when a hydrogen molecule spins about its centre of mass, it returns to the same location...
The figure shows plot of PV/T versus  for
for 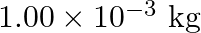 of oxygen gas at two different temperatures.
of oxygen gas at two different temperatures.
(a) What does the dotted plot signify? (b) Which is true: $\mathbf{T}_{1}>\mathbf{T}_{2}$ or $\mathbf{T}_{1}<\mathbf{T}_{2}$ ? Solution: (a) The dotted plot is perpendicular to the x-axis,...
One of the assumptions of the kinetic theory of gases is that there is no force of attraction between the molecules of a gas. State and explain the evidence that shows that the assumption is not applicable for real gases.
Under a condition of low pressure and high temperature the assumption made by kinetic theory is true. At high temperature, the molecules will be very far from each other and at low pressure, the...
Name two intermolecular forces that exist between HF molecules in a liquid state.
Hydrogen bonding and dipole-dipole interaction (HF-HF interaction) exists between HF molecule in a liquid state.
Name the energy which arises due to the motion of atoms or molecules in a body. How is this energy affected when the temperature is increased?
Thermal energy arises due to the motion of particles (atoms or molecules) in the body. If we increase the temperature then the kinetic energy of atom and molecule increases significantly and they...
The pressure exerted by saturated water vapour is called aqueous tension. What correction term will you apply to the total pressure to obtain a pressure of dry gas?
The total pressure of the gas is Pmoist gas = Pdry gas By applying the correction term, we have: Pdry gas = Pmoist gas – Aqueous tension Therefore, the correction term applied to the total pressure...
The magnitude of the surface tension of liquid depends on the attractive forces between the molecules. Arrange the following in increasing order of surface tension: Water, alcohol (C2H5OH) and hexane [CH3(CH2)4CH3)].
Hydrogen bonding is stronger in water than in alcohol. So, water has a strong intermolecular attraction than alcohol. The increasing order of surface tension is – Hexane< alcohol< water.
One of the assumptions of the kinetic theory of gases states that “there is no force of attraction between the molecules of a gas.” How far is this statement correct? Is it possible to liquefy an ideal gas? Explain.
The above statement is true. At a higher temperature the movement of gaseous molecules become faster such that there is no intermolecular attraction. Under this condition, gas behaves like an ideal...
Value of universal gas constant (R) is the same for all gases. What is its physical significance?
The dimension of the universal gas constant R is energy per degree per mole. In the metre-kilogram-second system, the value of R is 8.3144598 joules per Kelvin per mole. Hence R only depends on the...
Two different gases ‘A’ and ‘B’ are filled in separate containers of equal capacity under the same conditions of temperature and pressure. On increasing the pressure slightly the gas ‘A’ liquefies but gas B does not liquefy even on applying high pressure until it is cooled. Explain this phenomenon.
The critical temperature is the term used for this phenomenon. Here gas A liquefies means that A is below its critical temperature and gas B does not liquefy on applying high pressure as it is above...
A gas that follows Boyle’s law, Charles law and Avogadro’s law are called an ideal gas. Under what conditions a real gas would behave ideally?
All gases are not ideal gas. This means that they do not follow the above mentioned laws at all conditions of temperature, pressure or volume. Real gas doesn’t obey the gas law at normal temperature...
Use the information and data given below to answer the questions (a) to (c): • Stronger intermolecular forces result in a higher boiling point. • Strength of London forces increases with the number of electrons in the molecule. • Boiling point of HF, HCl, HBr and HI is 293 K, 189 K, 206 K and 238 K respectively. (a) Which type of intermolecular forces are present in the molecules HF, HCl, HBr and HI? (b) Looking at the trend of boiling points of HCl, HBr and HI, explain out of dipole-dipole interaction and London interaction, which one is predominant here. (c) Why is the boiling point of hydrogen fluoride highest while that of hydrogen chloride lowest?
(a) Since the halides are a polar molecule (due to high electronegativity), due to the presence of permanent dipoles, the dipole-dipole interactions along with the London forces are found in HF, HCl...
If 1 gram of each of the following gases are taken at STP, which of the gases will occupy (a) greatest volume and (b) smallest volume? CO, H2O, CH4, NO
(a) CH4 has the least molar mass (16gm), so it would occupy the highest volume for 1gm of the gas as higher the molar mass, the lesser is the volume occupied. (b) Similarly, NO has the highest...
Physical properties of ice, water and steam are very different. What is the chemical composition of water in all three states?
H2O exists in three different states of matter. It exists in the solid form as ice, in the liquid form as water and as steam in the gaseous state. All of these states consist of water, due to which...
Which of the following changes decrease the vapour pressure of water kept in a sealed vessel? (i) Decreasing the quantity of water (ii) Adding salt to the water (iii) Decreasing the volume of the vessel to one-half (iv) Decreasing the temperature of the water
The correct option is (ii) and (iv).
Under which of the following two conditions applied together, a gas deviates most from the ideal behaviour? (i) Low pressure (ii) High pressure (iii) Low temperature (iv) High temperature
The correct options are (ii) and (iii).
Estimate the fraction of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be 3 A.
Diameter of an oxygen molecule is given as $d=3 \AA$ Radius will be, $r=d / 2$ $r=3 / 2=1.5 \AA=1.5 \times 10^{-8} \mathrm{~cm}$ Actual volume occupied by 1 mole of oxygen gas at STP is given as...
With regard to the gaseous state of matter which of the following statements are correct? (i) Complete order of molecules (ii) Complete disorder of molecules (iii) Random motion of molecules (iv) Fixed position of molecules
Option (ii) and (iii) are the correct statements.
A man standing at a certain distance from an observer blows a horn of frequency 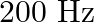 in still air.
in still air.
(a) Find the horn’s frequency for the observer when the man (i) runs towards him at 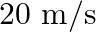 (ii) runs away from him at
(ii) runs away from him at 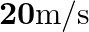 .
.
(b) Find the speed of sound in both the cases.
[Speed of sound in still air is ![Rendered by QuickLaTeX.com \mathbf{3 4 0 \mathrm { m } / \mathrm { s } \text { ] }}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f3e8ca26b9dfd0f318b50c1ded0b4984_l3.png)
Frequency of the horn is given as $\mathrm{v}_{\mathrm{H}}=200 \mathrm{~Hz}$ Velocity of the man is given as $\mathrm{v}_{\mathrm{T}}=20 \mathrm{~m} / \mathrm{s}$ Velocity of sound is given as...
Earthquakes generate sound waves inside the earth. Unlike a gas, the earth can experience both transverse (S) and longitudinal (P) sound waves. Typically the speed of the S wave is about 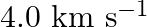 , and that of the
, and that of the  wave is
wave is 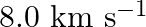 . A seismograph records
. A seismograph records  and
and 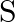 waves from an earthquake. The first P wave arrives 4 min before the first
waves from an earthquake. The first P wave arrives 4 min before the first  wave. Assuming the waves travel in a straight line, at what distance does the earthquake occur?
wave. Assuming the waves travel in a straight line, at what distance does the earthquake occur?
Let $S$ and $P$ have speeds of $v_{1}$ and $v_{2}$, respectively. The $S$ and $P$ waves take $t_{1}$ and $t_{2}$ seconds to reach the position of the seismograph, respectively. $I=v_{1} t_{1}=v_{2}...
A SONAR system fixed in a submarine operates at a frequency  kHz. An enemy submarine moves towards the SONAR with a speed of
kHz. An enemy submarine moves towards the SONAR with a speed of 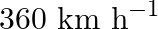 . What is the frequency of sound reflected by the submarine? Take the speed of sound in water to be
. What is the frequency of sound reflected by the submarine? Take the speed of sound in water to be 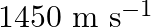 .
.
Frequency of the SONAR system is given as $\mathrm{f}=40 \mathrm{kHz}=40 \times 10^{3} \mathrm{~Hz}$ Speed of sound in water is given as $v=1450 \mathrm{~m} / \mathrm{s}$ Speed of the enemy...
One end of a long string of linear mass density 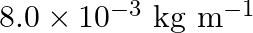 is connected to an electrically driven tuning fork of frequency
is connected to an electrically driven tuning fork of frequency  . The other end passes over a pulley and is tied to a pan containing a mass of
. The other end passes over a pulley and is tied to a pan containing a mass of 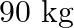 . The pulley end absorbs all the incoming energy so that reflected waves at this end have negligible amplitude. At
. The pulley end absorbs all the incoming energy so that reflected waves at this end have negligible amplitude. At  , the left end (fork end) of the string
, the left end (fork end) of the string 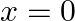 has zero transverse displacement
has zero transverse displacement 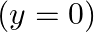 and is moving along positive
and is moving along positive 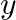 -direction. The amplitude of the wave is
-direction. The amplitude of the wave is 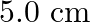 . Write down the transverse displacement y as a function of
. Write down the transverse displacement y as a function of 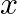 and
and 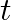 that describes the wave on the string.
that describes the wave on the string.
Linear mass density of the string is given as $\mu=8.0 \times 10^{-3} \mathrm{~kg} \mathrm{~m}^{-1}$ Frequency of the tuning fork is given as $=256 \mathrm{~Hz}$ Mass on the pan is given as $90...
A travelling harmonic wave on a string is described by 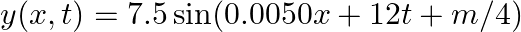
(a) What are the displacement and velocity of oscillation of a point at 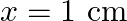 , and
, and 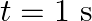 ? Is this velocity equal to the velocity of wave propagation?
? Is this velocity equal to the velocity of wave propagation?
(b) Locate the points of the string which have the same transverse displacements and velocity as the 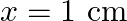 point at
point at 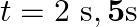 and
and 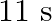
(a) The travelling harmonic wave is given by, $y(x, t)=7.5 \sin (0.0050 x+12 t+\pi / 4)$ At $x=1 \mathrm{~cm}$ and $\mathrm{t}=1 \mathrm{~s}$ $y(1,1)=7.5 \sin (0.0050(1)+12(1)+\pi / 4)$ $=7.5 \sin...
A train, standing at the outer signal of a railway station blows a whistle of frequency 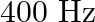 in still air. (i) What is the frequency of the whistle for a platform observer when the train (a) approaches the platform with a speed of
in still air. (i) What is the frequency of the whistle for a platform observer when the train (a) approaches the platform with a speed of 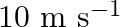 , (b) recedes from the platform with a speed of
, (b) recedes from the platform with a speed of 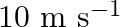 ? (ii) What is the speed of sound in each case? The speed of sound in still air can be taken as
? (ii) What is the speed of sound in each case? The speed of sound in still air can be taken as  .
.
Frequency of the whistle is given as $=400 \mathrm{~Hz}$ Speed of sound in still air is given as $=340 \mathrm{~m} / \mathrm{s}$ (i) (a)Train approaches the platform at a speed given as...
Explain how:
(i) A guitar note and violin note are being played at the same frequency, however, we can still make out which instrument is producing which note
(ii) Both transverse and longitudinal wave can propagate through solids, but only longitudinal waves can move through gases.
(i) Overtones are produced differently by the guitar and the violin. Even though the notes from a guitar and a violin vibrate at the same frequencies, it is possible to distinguish between them....
Explain how:
(i) A sound wave’s pressure antinode is a displacement node and vice versa.
(ii) The Ganges river dolphin despite being blind, can manoeuvre and swim around obstacles and hunt down preys.
(i) An antinode is a place where the pressure is lowest and the vibration amplitude is highest. A node, on the other hand, is a place where pressure is highest and vibration amplitude is lowest....
One end of A  long tube is closed. Find the harmonic mode of the tube that will be resonantly excited by a source of frequency
long tube is closed. Find the harmonic mode of the tube that will be resonantly excited by a source of frequency 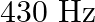 . If both the ends are open, can the same source still produce resonance in the tube? (Sound travels in air at
. If both the ends are open, can the same source still produce resonance in the tube? (Sound travels in air at 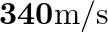 ).
).
Length of the pipe is given as $I=20 \mathrm{~cm}=0.2 \mathrm{~m}$ Frequency of the source is given as $\mathrm{n}^{\text {th }}$ Normal mode of frequency is given as $\mathrm{v}_{\mathrm{N}}=430...
A 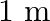 long pipe with a movable piston at one end and an opening at the other will be in resonance with a tuning fork vibrating at
long pipe with a movable piston at one end and an opening at the other will be in resonance with a tuning fork vibrating at 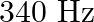 , if the length of the pipe is
, if the length of the pipe is 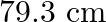 or
or 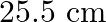 . Calculate the speed of sound in air. Neglect the edge effects.
. Calculate the speed of sound in air. Neglect the edge effects.
Frequency of the turning fork is given as $\mathrm{v}_{\mathrm{F}}=340 \mathrm{~Hz}$ Length of the pipe is given as $l_{1}=0.255 \mathrm{~m}$ Due to the presence of a piston at one end, the supplied...
Present below are functions of 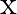 and
and 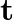 to describe the displacement (longitudinal or transverse) of an elastic wave. Identify the ones describing ( a ) a stationary wave, ( b ) a travelling wave and ( c) neither of the two:
to describe the displacement (longitudinal or transverse) of an elastic wave. Identify the ones describing ( a ) a stationary wave, ( b ) a travelling wave and ( c) neither of the two:
(i) 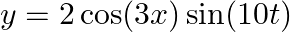
(ii) 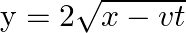
(i) Because the harmonic terms $\omega t$ and $k x$ exist individually, this equation depicts a stationary wave. (ii) There are no harmonic terms in this equation. As a result, it is neither a...
Considering the wave, 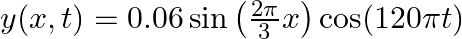
Answer the following questions;
(a) Are all the points in the wire oscillating at the same values of (i) frequency, (ii) phase, (iii) amplitude? Justify your answers.
(b) Calculate the amplitude of a point  away from one end?
away from one end?
(a) Because both ends of the wire are clamped, the ends act as nodes, and the entire wire vibrates as a single segment. Thus, (i) Except at the wire's ends, where the frequency is 0, all of the...
Atmospheric pressures recorded in different cities are as follows: Cities Shimla Bangalore Delhi Mumbai p in N/m2 1.01×105 1.2×105 1.02×105 1.21×105. Consider the above data and mark the place at which liquid will boil first. (i) Shimla (ii) Bangalore (iii) Delhi (iv) Mumbai
The correct option is (i) Shimla
The transverse displacement of a wire (clamped at both its ends) is described as : 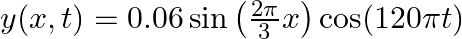 The mass of the wire is
The mass of the wire is  and its length is
and its length is 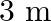 .
.
Provide answers to the following question:
Calculate the wire’s tension.
[X and 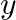 are in meters and
are in meters and 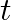 in secs]
in secs]
As we know, The standard equation of a stationary wave is known as, $y(x, t)=2 a \sin k x \cos w t$ Given equation is, $y(x, t)=0.06 \sin \left(\frac{2 \pi}{3} x\right) \cos (120 \pi t)$ It is...
The transverse displacement of a wire (clamped at both its ends) is described as : 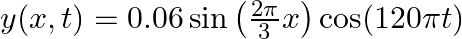 The mass of the wire is
The mass of the wire is 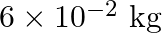 and its length is
and its length is 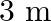 .
.
Provide answers to the following questions:
(i) Is the function describing a stationary wave or a travelling wave?
(ii) Interpret the wave as a superposition of two waves travelling in opposite directions. Find the speed, wavelength and frequency of each wave.
As we know, The standard equation of a stationary wave is known as, $y(x, t)=2 a \sin k x \cos w t$ Given equation is, $y(x, t)=0.06 \sin \left(\frac{2 \pi}{3} x\right) \cos (120 \pi t)$ It is...
What is the SI unit of viscosity coefficient (η)? (i) Pascal (ii) Nsm–2 (iii) km–2 s (iv) N m–2
The correct option is (ii) Nsm–2
Gases possess characteristic critical temperature which depends upon the magnitude of intermolecular forces between the particles. Following are the critical temperatures of some gases. Gases H2 He O2 N2 Critical temperature in Kelvin 33.2 5.3 154.3 126 From the above data what would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first (i) H2, He, O2, N2 (ii) He, O2, H2, N2 (iii) N2, O2, He, H2 (iv) O2, N2, H2, He
The correct option is (iv) O2, N2, H2, He.
A travelling harmonic wave is given as: 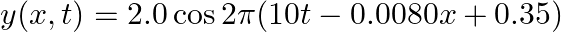 . What is the phase difference between the oscillatory motion of two points separated by a distance of:
. What is the phase difference between the oscillatory motion of two points separated by a distance of:
(i) 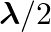 ,
,
(ii) 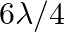
[  and
and  are in
are in  and
and 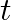 is in secs
is in secs ![Rendered by QuickLaTeX.com ]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-478d84af1027ee3593251b323a3fb181_l3.png) .
.
Equation for a travelling harmonic wave is given as, $\begin{array}{l} y(x, t)=2.0 \cos 2 \pi(10 t-0.0080 x+0.35) \\ =2.0 \cos (20 \pi t-0.016 \pi x+0.70 \pi) \end{array}$ where, Propagation...
As the temperature increases, the average kinetic energy of molecules increases. What would be the effect of the increase of temperature on pressure provided the volume is constant? (i) increases (ii) decreases (iii) remains the same (iv) becomes half
The correct option is (i) increases.
A travelling harmonic wave is given as: 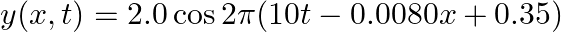 . What is the phase difference between the oscillatory motion of two points separated by a distance of:
. What is the phase difference between the oscillatory motion of two points separated by a distance of:
(i) 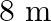 ,
,
(ii) 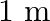
[ 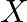 and
and 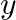 are in
are in  and
and  is in secs
is in secs ![Rendered by QuickLaTeX.com ]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-478d84af1027ee3593251b323a3fb181_l3.png) .
.
Equation for a travelling harmonic wave is given as, $\begin{array}{l} y(x, t)=2.0 \cos 2 \pi(10 t-0.0080 x+0.35) \\ =2.0 \cos (20 \pi t-0.016 \pi x+0.70 \pi) \end{array}$ where, Propagation...
The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen? (i) 0.8×105 atm (ii) 0.008 Nm–2 (iii) 8×104 Nm–2 (iv) 0.25 atm
The correct option is (iii) 8×104 Nm–2
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial charges’. The partial charge is (i) more than unit electronic charge (ii) equal to unit electronic charge (iii) less than unit electronic charge (iv) double the unit electronic charge
The correct option is (iii) less than unit electronic charge.
A transverse harmonic wave on a wire is expressed as: 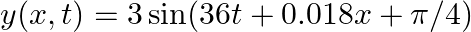
Calculate the smallest distance between two adjacent crests in the wave.
[ and
and  are in
are in  and
and 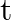 in seconds. Assume the left to right direction as the positive direction of
in seconds. Assume the left to right direction as the positive direction of ![Rendered by QuickLaTeX.com \mathrm{x}]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7eec0f19e3a8a19059d43c95ee5abebd_l3.png)
Given function is, $(x, t)=3 \sin (36 t+0.018 x+\pi / 4)$ The smallest distance between two adjacent crests in the wave can be calculated as, $\lambda=2 \pi / \mathrm{k}=2 \mathrm{~m} / 0.018$ $=349...
A transverse harmonic wave on a wire is expressed as: 
i) Find its frequency and amplitude.
ii) Give the initial phase at the origin.
[ and
and  are in
are in 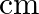 and
and 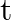 in seconds. Assume the left to right direction as the positive direction of
in seconds. Assume the left to right direction as the positive direction of ![Rendered by QuickLaTeX.com \mathrm{x}]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7eec0f19e3a8a19059d43c95ee5abebd_l3.png)
Given function is, $(x, t)=3 \sin (36 t+0.018 x+\pi / 4)$ i) Amplitude of the wave is given as $a=3 \mathrm{~cm}$ Frequency of the wave can be calculated as $v=\omega / 2 \pi$ $36 / 2 \pi=5.7...
An ultrasonic scanner operating at 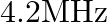 is used to locate tumours in tissues. If the speed of sound is
is used to locate tumours in tissues. If the speed of sound is 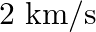 in a certain tissue, calculate the wavelength of sound in this tissue.
in a certain tissue, calculate the wavelength of sound in this tissue.
Speed of sound in the tissue is given as, $v_{T}=2 \mathrm{~km} / \mathrm{s}=2 \times 10^{3} \mathrm{~m} / \mathrm{s}$ Operating frequency of the scanner is given as $\mathrm{v}=4.2 \mathrm{MHz}=4.2...
A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason for this observation is that at high altitude: (i) pressure increases (ii) temperature decreases (iii) pressure decreases (iv) temperature increases
The correct option is (iii) pressure decreases.
We know that the function 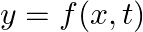 represents a wave travelling in one direction, where
represents a wave travelling in one direction, where  and
and  must appear in the combination
must appear in the combination 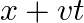 or
or 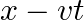 or i.e.
or i.e. 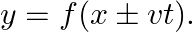 Is the converse true? Can the following functions for y possibly represent a travelling wave:
Is the converse true? Can the following functions for y possibly represent a travelling wave:
(i)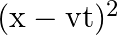
(ii) ![Rendered by QuickLaTeX.com \log \left[(x+v t) / x_{0}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-99d2bcfc07e0da1a668137fffad738d8_l3.png)
(iii) 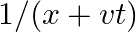
No, the opposite is not true, because a wave function representing a travelling wave must have a finite value for all $x$ and $t$ values. None of the functions above satisfy the criteria, hence none...
Using the formula 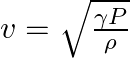 explain why the speed of sound in air increases with humidity.
explain why the speed of sound in air increases with humidity.
Given values are, $v=\sqrt{\frac{\gamma P}{\rho}}$ $\Rightarrow \mathrm{P}=\frac{\rho R T}{M}$ $\Rightarrow \frac{P}{\rho}=\frac{R T}{M}$ The effective density of the air decreases as humidity...
Using the formula 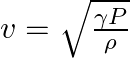 explain why the speed of sound in air
explain why the speed of sound in air
(a) does not depend upon pressure.
(b) increases with temperature and humidity.
Given values are, $v=\sqrt{\frac{\gamma P}{\rho}}$ $\Rightarrow \mathrm{P}=\frac{\rho R T}{M}$ $\Rightarrow \frac{P}{\rho}=\frac{R T}{M}$ (a) For a gas at a constant temperature, $\frac{P}{\rho}=...
Which of the following statements is correct? (i) Metallic hydrides are deficient of hydrogen. (ii) Metallic hydrides conduct heat and electricity. (iii) Ionic hydrides do not conduct electricity in solid-state. (iv) Ionic hydrides are very good conductors of electricity in solid-state.
Solution: Option (i), (ii) and (iii) are the answers. Metallic hydrides are hydrides that are not stoichiometric in nature. They are excellent conductors of heat and electricity. Ionic hydrides...
A stone dropped from the top of a tower of height 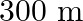 splashes into the water of a pond near the base of the tower. When is the splash heard at the top given that the speed of sound in air is
splashes into the water of a pond near the base of the tower. When is the splash heard at the top given that the speed of sound in air is 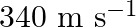 ?
? 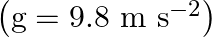
Height of the bridge is given as $s=300 \mathrm{~m}$ Initial velocity of the stone is given as $u=0$ Acceleration is known as $a=g=9.8 \mathrm{~m} / \mathrm{s}^{2}$ Speed of sound in air is given as...
