The monomer in this case is –A-. This polymer is a homopolymer since the same or same monomer is repeated to make it.
A natural linear polymer of 2-methyl-1, 3-butadiene becomes hard on treatment with sulphur between 373 to 415 K and —S—S— bonds are formed between chains. Write the structure of the product of this treatment?
The structure of 2-methyl-1,3-butadiene is Rubber vulcanization is the term for this procedure. Sulphur works as a crosslinking agent in this case, stiffening the rubber and giving it better...
Vulcanisation makes rubber ______________. (i) more elastic (ii) soluble in inorganic solvent (iii) crystalline (iv) more stiff
Option (i) and (iv) are the answers. Rubber becomes more elastic and rigid after vulcanization. Sulphur produces cross connections at the reactive locations of double bonds or at their reactive...
Which of the following polymers have vinylic monomer units? (i) Acrilan (ii) Polystyrene (iii) Nylon (iv) Teflon
Option (i), (ii) and (iv) are the answers. The vinylic monomer units of acrilan, polystyrene, and teflon are as follows:
Which of the following polymers can have strong intermolecular forces? (i) Nylon (ii) Polystyrene (iii) Rubber (iv) Polyesters
Option (i) and (iv) are the answers. Nylon and polyester are thread-forming fibres with a high melting point and tensile strength. Intermolecular forces such as hydrogen bonding are strong.
Which of the following is an example of a synthetic rubber? (i) Polychloroprene (ii) Polyacrylonitrile (iii) Buna-N (iv) cis-polyisoprene
Option (i) and (iii) are the answers. Polychloroprene and Buna-N are examples of a synthetic rubber
Which of the following monomers form biodegradable polymers? (i) 3-hydroxybutyric acid + 3-hydroxypentanoic acid (ii) Glycine + aminocaproic acid (iii) Ethylene glycol + phthalic acid (iv) Caprolactam
Option (i) and (ii) are the answers. Biodegradable polymers are those that are quickly degraded and are not hazardous to the environment.
Which of the following polymers are condensation polymers? (i) Bakelite (ii) Teflon (iii) Butyl rubber (iv) Melamine formaldehyde resin
Option (i) and (iv) are the answers. Bakelite is a phenol and formaldehyde condensation polymer. Melamine formaldehyde resin is a melamine (2, 4, 6-triamino-1, 3, 5-triazine) and formaldehyde...
Which of the following are addition polymers? (i) Nylon (ii) Melamine formaldehyde resin (iii) Orlon (iv) Polystyrene
Option (iii) and (iv) are the answers. The addition polymers Orion and polystyrene are produced by polymerizing CH2 = CH – CN (acrylonitrile) and C6H5 – CH = CH2 styrene, respectively.
Which of the following polymers are used as fibre? (i) Polytetrafluoroethane (ii) Polychloroprene (iii) Nylon (iv) Terylene
Option (iii) and (iv) are the answers. Because of strong intermolecular interactions such as H-bonding, nylon and terylene are employed as fibres, resulting in tight chain packing and therefore...
Which of the following polymers are thermoplastic? (i) Teflon (ii) Natural rubber (iii) Neoprene (iv) Polystyrene
Option (i) and (iv) are the answers. Teflon and polystyrene are thermoplastics because they can be melted and moulded anew.
Which of the following are characteristics of thermosetting polymers? (i) Heavily branched cross-linked polymers. (ii) Linear slightly branched long-chain molecules. (iii) Become infusible on moulding so cannot be reused. (iv) Soften on heating and harden on cooling, can be reused.
Option (i) and (iii) are the answers. Thermosetting polymers have a lot of branching cross links. They can't be used again since they don't melt when heated and can't be remoulded.
Which of the following polymers, need at least one diene monomer for their preparation? (i) Dacron (ii) Buna-S (iii) Neoprene (iv) Novolac
Option (ii) and (iii) are the answers. (b) (c)
Which of the following polymer can be formed by using the following monomer unit?
Option (iv) is the answer. Nylon -6 is a polymer made from caprolactam polymerization.
is a polymer having monomer units ____________.
Option (i) is the answer.
Which of the following statements is not true about low-density polythene? (i) Tough (ii) Hard (iii) Poor conductor of electricity (iv) Highly branched structure
Option (ii) is the answer. Low density polythene is a strong yet flexible (not too flexible) material with a weak electrical conductivity. Its structure is extremely branching.
In which of the following polymers ethylene glycol is one of the monomer units?
Option (i) is the answer. Condensation polymerization of ethylene glycol and phthalic acid with the removal of the water molecule yields the given polymer.
Which of the following polymer is biodegradable?
Option (C) is the answer. Biodegradable polymers are materials whose chemical and physical properties deteriorate and disintegrate entirely when exposed to microorganisms, aerobic, and anaerobic...
The commercial name of polyacrylonitrile is ______________. (i) Dacron (ii) Orlon (Acrilan) (iii) PVC (iv) Bakelite
Option (ii) is the answer.
Which of the following is not a semisynthetic polymer? (i) cis-polyisoprene (ii) Cellulose nitrate (iii) Cellulose acetate (iv) Vulcanised rubber
Option (i) is the answer M-polyisoprene is a polymer that comes from nature.
Which of the following polymers of glucose is stored by animals? (i) Cellulose (ii) Amylose (iii) Amylopectin (iv) Glycogen
Option (iv) is the answer. Glycogen is a glucose polymer present in animal livers, brains, and muscles. Amylase and amylopectin are structural components of starch, while cellulose is a polymer...
Compound ‘A’ with molecular formula C4H9Br is treated with aq. KOH solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. KOH solution, the rate of reaction was found to be dependent on the concentration of compound and KOH both. (i) Write down the structural formula of both compounds ‘A’ and ‘B’. (ii) Out of these two compounds, which one will be converted to the product with an inverted configuration.
Because the rate of reaction of compound ‘A' (C4H9Br) with aqueous KOH is solely determined by the concentration of compound ‘A,' the reaction happens via the SN1 mechanism, and product ‘A' is...
Classify the following compounds as primary, secondary and tertiary halides. (i) 1-Bromobut-2-ene (ii) 4-Bromopent-2-ene (iii) 2-Bromo-2-methylpropane
(i)A main halide is 1-bromobut-2-ene. (ii) Bromine is linked to the secondary carbon atom in 4-Bromopent-2-ene, making it a secondary halide. (iii) Bromine is linked to the tertiary carbon atom in...
Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.
Because electron density is higher at ortho and para locations, the functional groups contained in these compounds are ortho-para directed.
Why is the solubility of haloalkanes in water very low?
Because energy is required to overcome the attractions between the haloalkane molecules as well as to break the hydrogen bonds between water molecules, haloalkanes are only weakly soluble in water.
Which of the products will be a major product in the reaction given below? Explain. CH3CH=CH2 + HI → CH3CH2CH2I + CH3CHICH30 (A) (B)
The molecule (B) will be the reaction's main product. This addition reaction is carried out according to Markovnikoff's rule, in which the hydrogen from the hydrogen halide is added to the carbon...
Which of the following compounds (a) and (b) will not react with a mixture of NaBr and H2SO4. Explain why?
Br2 gas is produced when NaBr and H2SO4 are combined. Because of the stable molecule created as a result of resonance stabilisation, molecule (b) will not react with Br2 gas.
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Electrophilic substitution can be used to make aryl bromides and chlorides from arenes. In the absence of light, this reaction is carried out by treating the arene with chlorine or bromine in the...
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
This is due to the aryl ring's resonance stabilisation. There will be a conjugation of chlorine electrons with the electrons in the ring in the case of C6H5-Cl. The partial double bond nature of the...
Why iodoform has appreciable antiseptic property?
Iodoform has a significant antibacterial activity due to the release of free iodine.
Which of the compounds will react faster in SN1 reaction with the –OH ion? CH3— CH2— Cl or C6H5— CH2— Cl
In an SN1 reaction with the OH- ion, C6H5— CH2— Cl will react quicker. This is owing to the carbocation's stability in the compound. The C6H5 group is already stable owing to resonance, and the CH2...
Out of o-and p-bromobenzene which one has a higher melting point and why?
Because the symmetry of p-dibromobenzene allows the molecule to fit better in a crystal lattice, it has a higher melting point. As a result, breaking the bonds between the molecules needs a greater...
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does the preparation of aryl iodides requires the presence of an oxidising agent?
Arenes' iodination can be reversed due to the production of HI. An oxidising agent, such as HNO3 or HIO4, oxidises HI to speed up the process and stabilise the result.
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ____________ or ____________. (i) Ca F2 (ii) CoF2 (iii) Hg2F2 (iv) NaF
Option (ii) and (iii) are the answers. Heating an alkyl chloride or bromide in the presence of a metallic fluoride such as AgF, FIg2F2, CoF2, or SbF3 results in the production of alkyl fluorides....
Alkyl halides are prepared from alcohols by treating with (i) HCl + ZnCl2 (ii) Red P + Br2 (iii) H2SO4+ KI (iv) All the above
Option (i) and (ii) are the answers (i) $R-\mathrm{OH} \stackrel{\mathrm{HCl}+\mathrm{ZnCl}}{\longrightarrow} R-\mathrm{Cl}_{2}+\mathrm{H}_{2} \mathrm{O}$ (ii) $R-\mathrm{OH}...
Which of the following compounds can be classified as aryl halides? (i) p-ClC6H4CH2CH(CH3)2 (ii) p-CH3CHCl(C6H4)CH2CH3 (iii) o-BrH2C-C6H4CH(CH3)CH2CH3 (iv) C6H5-Cl
Option (i) and (iv) are the answers. Because the halogen atom is directly linked to the aromatic ring in compounds (i) and (iv), these compounds are classed as aryl halides.
Which of the following are secondary bromides? (i) (CH3)2 CHBr (ii) (CH3)3C CH2Br (iii) CH3CH(Br)CH2CH3 (iv) (CH3)2CBrCH2CH3
Option (i) and (iii) are the answers. Secondary bromides are those in which the -carbone (bromine-bound carbon) is further attached to two alkyl groups. The -carbon in compounds (i) and (iii) is...
Which of the following compounds are gem-dihalides? (i) Ethylidene chloride (ii) Ethylene dichloride (iii) Methylene chloride (iv) Benzyl chloride
Option (i) and (iii) are the answers. Dihalides with two halogen atoms linked to the same carbon atom are known as gem-dihalides. Gem-dihalides are formed when two halogen atoms are present on the...
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements. (i) Both the compounds form the same product on treatment with alcoholic KOH. (ii) Both the compounds form the same product on treatment with aq.NaOH. (iii) Both the compounds form the same product on reduction. (iv) Both the compounds are optically active.
Option (i) and (iii) are the answers. They give ethyne on treatment with alcoholic $\mathrm{KOH}$. $ \mathrm{CH}_{3} \mathrm{CHCl}_{2} \underset{\mathrm{KOH}}{\stackrel{\text { alc....
Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds. (i) 2-Bromopentane (ii) Vinyl chloride (chloroethene) (iii) 2-chloroacetophenone (iv) Trichloromethane
Option (i) and (iv) are the answers. Halogen atoms are linked to the sp3 hybridised carbon atom of the alkyl group in each of these molecules.
Which of the following statements are correct about the kinetics of this reaction? (i) The rate of reaction depends on the concentration of only (b). (ii) The rate of reaction depends on the concentration of both (a) and (b). (iii) Molecularity of reaction is one. (iv) Molecularity of reaction is two.
Option (i) and (iii) are the answers. The SN1 mechanism is used in the given reaction. The production of carbocation is a gradual process in the SN1 mechanism. As a result, the pace of reaction is...
Which of the following statements are correct about the mechanism of this reaction? (i) A carbocation will be formed as an intermediate in the reaction. (ii) OH–will attach the substrate (b) from one side and Cl- will leave it simultaneously from the other side. (iii) An unstable intermediate will be formed in which OH– and Cl– will be attached by weak bonds. (iv) The reaction proceeds through an SN1 mechanism.
Option (i) and (iv) are the answers. Because it is a tertiary halide, it undergoes the SN1 process, resulting in the formation of a carbocation as an intermediate.
Which of the following statements are correct about the reaction intermediate? (i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms. (ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised. (iii) Intermediate (c) is stable because carbon atom is sp2 hybridised. (iv) Intermediate (c) is less stable than the reactant (b).
Option (i) and (iv) are the answers. Intermediate (iii) is unstable in the above reaction because the carbon atom is linked to 5 atoms and is less stable than reactant (ii).
Which of the following statements are correct about this reaction? (i) The given reaction follows the SN2 mechanism. (ii) (b) and (d) have the opposite configuration. (iii) (b) and (d) have the same configuration. (iv) The given reaction follows the SN1 mechanism.
Option (i) and (ii) are the answers. Alkyl halide is the main reactant in the given reaction. A transient condition is found here, in which one bond is broken and another is created synchronously,...
Which of the statements are correct about the above reaction? (i) (a) and (e) both are nucleophiles. (ii) In (c) carbon atom is sp3 hybridised. (iii) In (c) carbon atom is sp2 hybridised. (iv) (a) and (e) both are electrophiles.
Option (i) and (iii) are the answers. Nucleophiles are HO and CF. The C atom is sp2 hybridised in (iii) due to the simultaneous creation of the C – OH bond and the breakdown of the C – Cl link. As a...
Which is the correct increasing order of boiling points of the following compounds? 1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene (i) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane (ii) Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane (iii) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene (iv) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Option (iv) is the answer Reason: As the molecular mass of the alkyl halide grows, the boiling point rises.
Which is the correct increasing order of boiling points of the following compounds? 1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane (i) Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane (ii) 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane (iii) Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane (iv) Butane < 1-Chlorobutane < 1-Iodobutane < 1-BromobutaneSolution:
Option (i) is the answer. Explanation: The larger the surface area, the stronger the intermolecular forces of attraction and, as a result, the higher the boiling point. For comparable types of alkyl...
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (b) < (a) < (c) (iii) (c) < (b) < (a) (iv) (a) < (c) < (b)
Option (iii) is the answer. The amount of electron releasing groups increases the reactivity of aryl halides; the fewer the electron releasing groups, the slower the rate of nucleophilic...
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (c) < (b) < (a) (ii) (b) < (c) < (a) (iii) (a) < (c) < (b) (iv) (a) < (b) < (c)
Option (iv) is the answer. Electron withdrawing groups boost aryl halide reactivity; the higher the number of electron withdrawing groups, the higher the rate of nucleophilic substitution.
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (a) < (c) < (b) (iii) (c) < (b) < (a) (iv) (b) < (c) < (a)
Option (iv) is the answer. The presence of an electron-releasing group in the ortho or para locations slows down nucleophilic substitution.
arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.(i) (a) < (b) < (c) (ii) (c) < (b) < (a) (iii) (a) < (c) < (b) (iv) (c) < (a) < (b)
Option (iii) is the answer. Nucleophilic substitution is facilitated by the presence of an electron withdrawing group (-NO2) in the ortho and para positions. At meta position, the presence of an...
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH– ion?(i) (a) (ii) (a), (b), (c) (iii) (b), (c) (iv) (a), (c)
Option (i) is the answer. At least one chiral carbon must be present in the molecule to display racemic mixing. Chiral carbon is an asymmetric carbon that is linked to four distinct sorts of atoms...
Which of the carbon atoms present in the molecule given below are asymmetric?(i) a, b, c, d (ii) b, c (iii) a, d (iv) a b, c
Option (ii) is the answer. The chiral carbon, which is $sp_3$ hybridised carbon attached to four distinct substituents, is known as asymmetric carbon. Because (b) and (c) are $sp_3$ hybridised, they...
Reaction of C6H5CH2Br with aqueous sodium hydroxide follows ____________. (i) SN1 mechanism (ii) SN2 mechanism (iii) Any of the above two depending upon the temperature of the reaction (iv) Saytzeff rule
Option (i) is the answer. The $S_N$ 1 mechanism operates in watery media. As a result of this reaction, a stable carbocation forms as an intermediate.
Molecules whose mirror image is non-superimposable over them are known as chiral. Which of the following molecules is chiral? (i) 2-Bromobutane (ii) 1-Bromobutane (iii) 2-Bromopropane (iv) 2-Bromopropan-2-ol
Chiral molecules are those that lack a plane of symmetry as well as a centre of symmetry.. It is a chiral molecule because it lacks the plane of symmetry and the centre of symmetry. Option (i) is...
Chloromethane on treatment with an excess of ammonia yields mainly
Option (iii) is the answer. After reacting with $NH_3$ and chloromethane, methanamine is formed.
The reaction of toluene with chlorine in the presence of iron and the absence of light yields ____________.
Option (d) is the answer. In the presence of iron and in the absence of light, toluene reacts with chlorine to produce a mixture of 1-chloro-2-methylbenzene and 1-chloro-4-methylbenzene. The methyl...
What should be the correct IUPAC name for diethylbromomethane? (i) 1-Bromo-1,1-diethylmethane (ii) 3-Bromopentane (iii) 1-Bromo-1-ethylpropane (iv) 1-Bromopentane
Option (ii) is the answer. Diethylbromomethane's proper IUPAC designation is 3 - Bromopentane. $C_5H_{11}Br$ is the chemical formula for it.
Which is the correct IUPAC name for? (i) 1-Bromo-2-ethylpropane (ii) 1-Bromo-2-ethyl-2-methylethane (iii) 1-Bromo-2-methylbutane (iv) 2-Methyl-1-bromobutane
Option (iii) is the answer. correct IUPAC name for given compound is 1-Bromo-2-methylbutane
Which of the following alkyl halides will undergo SN1 reaction most readily? (i) (CH3)3C—F (ii) (CH3)3C—Cl (iii) (CH3)3C—Br (iv) (CH3)3C—I
Option (iv) is the answer. SN1 reactions occur mostly in polar protic solvents such as H2O and follow first-order kinetics. This indicates that the reaction rate is solely determined by one...
A primary alkyl halide would prefer to undergo _____________. (i) SN1 reaction (ii) SN2 reaction (iii) α–Elimination (iv) Racemisation
Option (ii) is the answer. Primary alkyl halides undergo SN2 mechanisms because 1∘ substrates have little steric hindrance to nucleophilic attack and 1∘ carbocations are relatively unstable....
What is ‘A’ in the following reaction?
The solution is option (iii). The addition of HCl across the double bond follows the Markovnikov rule in the aforementioned reaction. The negative portion of the addition molecule is linked to the...
Ethylidene chloride is a/an ______________. (i) vic-dihalide (ii) gem-dihalide (iii) allylic halide (iv) vinylic halide
Option (ii) is the answer. A gem-dihalide is ethylidene chloride. A compound with two halogen atoms on the same carbon atom is known as Gem Dihalide.
Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of 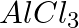 . Which of the following species attacks the benzene ring in this reaction? (i) Cl– (ii) Cl+ (iii)
. Which of the following species attacks the benzene ring in this reaction? (i) Cl– (ii) Cl+ (iii) 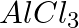 (iv) [AlCl4]–
(iv) [AlCl4]–
Option (ii) is the answer. Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of AlCl3. Cl+ attacks the benzene ring in this reaction.
The position of –Br in the compound in CH3CH==CHC(Br)(CH3)2 can be classified as ____________. (i) Allyl (ii) Aryl (iii) Vinyl (iv) Secondary
Optuion (i) is the answer. A) Allyl : $\mathrm{CH}_{3} \mathrm{CH}=\mathrm{CHC}(\mathrm{Br})\left(\mathrm{CH}_{3}\right)_{2}$ B) Aryl: $\mathrm{p}-\mathrm{ClC}_{6} \mathrm{H}_{4} \mathrm{CH}_{2}...
Which of the following is an example of vic-dihalide? (i) Dichloromethane (ii) 1,2-dichloroethane (iii) Ethylidene chloride (iv) Allyl chloride
Option (ii) is the answer. Vicinal dihalides are formed when a halogen reacts with an alkene to form compounds with halogen on neighbouring carbons. In terms of yearly output, 1, 2- dichloroethane...
Which of the following structures is enantiomeric with the molecule (A) given below :
Option (i) is the answer. Enantiomers are a pair of molecules that are mirror copies of one another.
In which of the following molecules carbon atom marked with an asterisk (*) is asymmetric?(i) (a), (b), (c), (d) (ii) (a), (b), (c) (iii) (b), (c), (d) (iv) (a), (c), (d)
Option (ii) is the answer. Asymmetric carbon atoms are those that have four distinct groups or atoms connected to them. The compounds a,b,c satisfy this criterion, thus B is the right answer.
Arrange the following compounds in increasing order of their boiling points.(i) (b) < (a) < (c) (ii) (a) < (b) < (c) (iii) (c) < (a) < (b) (iv) (c) < (b) < (a)
Option (iii) is the answer. The boiling point of an alkyl halide falls as the branching rises. As a result, tert butyl bromide has the lowest boiling point while n butyl bromide has the greatest...
Arrange the following compounds in the increasing order of their densities.(i) (a) < (b) < (c) < (d) (ii) (a) < (c) < (d) < (b) (iii) (d) < (c) < (b) < (a) (iv) (b) < (d) < (c) < (a)
Option (i) is the answer. Reason:The density of a substance is exactly proportional to its molar mass at constant volume.
Which reagent will you use for the following reaction? CH3CH2CH2CH3 → CH3CH2CH2CH2Cl + CH3CH2CHClCH3 (i) Cl2/UV light (ii) NaCl + H2SO4 (iii) Cl2 gas in dark (iv) Cl2 gas in the presence of iron in dark
Option (i) is the answer. Mono-chlorinated isomeric products of Cl 2/UV light free radical substitution
Which of the following is the halogen exchange reaction?
Option (i) is the answer. Metal–halogen exchange is a basic reaction in organometallic chemistry that transforms an organic halide into an organometallic product. Electropositive metals (Li, Na, Mg)...
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is (i) Electrophilic elimination reaction (ii) Electrophilic substitution reaction (iii) Free radical addition reaction (iv) Nucleophilic substitution reaction
Option (ii) is the answer. In the presence of Iron(III) chloride, toluene interacts with halogen to produce ortho and para halo compounds in an electrophilic substitution process. The Cl atom...
Identify the compound Y in the following reaction.
Option (i) is the answer.
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Option (iv) is the answer. If any alkyl halide forms a more stable carbocation at room temperature, the result is a more stable and efficient product. The formation of a $3^0$ carbocation as an...
The order of reactivity of following alcohols with halogen acids is ___________.(i) (A) > (B) > (C) (ii) (C) > (B) > (A) (iii) (B) > (A) > (C) (iv) (A) > (C) > (B)
Option (ii) is the answer. Alcohol reactivity towards halogen acids diminishes in the following order: (C) > (B) > (A) Because tertiary carbocation is the most stable of the three, it is...
Which of the following are benzylic alcohols?
Option (ii) and (iii) are the answers. Benzylic alcohols, as we all know, are molecules with an alcohol functional group on a carbon atom that is directly linked to the Benzene ring. Because the –OH...
Phenol can be distinguished from ethanol by the reactions with _________. (i) Br2/water (ii) Na (iii) Neutral FeCl3 (iv) All the above
Option (i) and (iii) are the answers. Phenols react with FeCl3 to create a colourful Fe3+ ion complex. Depending on the structure of the phenol examined, the hue ranges from purple to orange....
Which of the following reagents can be used to oxidise primary alcohols to aldehydes? (i) CrO3 in an anhydrous medium. (ii) KMnO4 in acidic medium. (iii) Pyridinium chlorochromate. (iv) Heat in the presence of Cu at 573K
Option (i), (iii) and (iv) are the answers. Primary alcohols can be oxidised to aldehydes using CrO3 in an anhydrous media. CrO3 serves as an oxidizer in this situation. As a result, option I is the...
Which of the following reactions will yield phenol?
Option (A), (B) and (C) are correct Diazonium salt is produced when aniline is treated with NaNO2 + HCl. The Diazonium salts are then hydrolyzed to Phenols by heating them with water. As a result,...
Which of the following are used to convert RCHO into RCH2OH? (i) H2/Pd (ii) LiAlH4 (iii) NaBH4 (iv) Reaction with RMgX followed by hydrolysis
Option (i), (ii) and (iii) are the answers. In the presence of a catalyst such as Pd, Pt, or Ni, aldehydes can be transformed to their corresponding alcohols by hydrogen. Lithium aluminium hydride...
Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol (i) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol (ii) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol (iii) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol (iv) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Option (i) is the answer As the moleculer mass of the alcohol grows, the boiling point rises. Furthermore, $1^0$ alcohols have greater boiling points than $2^0$ alcohols among isomeric alcohols.
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.(i) a < b < c (ii) b < a < c (iii) b < c < a (iv) c < b < a
Option (iii) is the answer. It's a nucleophilic substitution process that uses the $SN_1$ mechanism. The stability of the carbocation is required for the $SN_1$ mechanism to work. The presence of an...
Mark the correct order of decreasing acid strength of the following compounds.(i) e > d > b > a > c (ii) b > d > a > c > e (iii) d > e > c > b > a (iv) e > d > c > b > a
Option (ii) is the answer. The sequence of decreasing acid strength is b>d>a>c>e, with p-nitrophenol being the most acidic and p-methoxy phenol being the least acidic. The acidity is...
Which of the following is most acidic? (i) Benzyl alcohol (ii) Cyclohexanol (iii) Phenol (iv) m-Chlorophenol
Option (iv) is the answer. Alcohols have a lower acidity level than phenol. Furthermore, electron-withdrawing groups make phenols more acidic, therefore m-chlorophenol is the most acidic.
Phenol is less acidic than ______________. (i) ethanol (ii) o-nitrophenol (iii) o-methyl phenol (iv) o-methoxy phenol
Option (ii) is the answer. Because electron withdrawing $-NO_2$ groups enhance the acidity of phenols, while electron donating groups ($-CH_3$,$-OCH_3$) lower the acidity of phenols, phenol is less...
Which of the following compounds will react with sodium hydroxide solution in water? (i) C6H5OH (ii) C6H5CH2OH (iii) (CH3)3 COH (iv) C2H5OH
Option (i) is the answer Because phenols are more acidic than alcohols, they will react with a sodium hydroxide solution in water.
Which of the following species can act as the strongest base?
Option (ii) is the answer. The conjugate base of the weakest acid is the strongest. Because R OH is the weakest acid, $RO^-$ is the most powerful base.
IUPAC name of the compound is ______________.(i) 1-methoxy-1-methylethane (ii) 2-methoxy-2-methylethane (iii) 2-methoxypropane (iv) isopropylmethyl ether
Option (iii) is the answer. 2methoxypropane is the IUPAC name for the compound.
IUPAC name of m-cresol is ___________. (i) 3-methylphenol (ii) 3-chlorophenol (iii) 3-methoxyphenol (iv) benzene-1,3-diol
Option (i) is the answer. Because OH has a greater priority, its position on the benzene ring is number one, and the methyl group with locant number three is meta to it. 3-Methylphenol will be its...
Give IUPAC name of the compound given below.(i) 2-Chloro-5-hydroxyhexane (ii) 2-Hydroxy-5-chlorohexane (iii) 5-Chlorohexan-2-ol (iv) 2-Chlorohexan-5-ol
Option (iii) is the answer. We begin by choosing the longest carbon chain in the supplied organic molecule, as defined by IUPAC nomenclature. We use hexane because the longest carbon chain has six...
Which of the following compounds is aromatic alcohol?(i) A, B, C, D (ii) A, D (iii) B, C (iv) A
Option (iii) is the answer. Aromatic alcohols are those in which the -OH group is not directly connected to the benzene ring. As a result, option C is accurate.
The process of converting alkyl halides into alcohols involves_____________. (i) addition reaction (ii) substitution reaction (iii) dehydrohalogenation reaction (iv) rearrangement reaction
Option (ii) is the answer. Since the $X^-$ group is replaced by the $OH^-$ group, the process of turning alkyl halides into alcohols may be written as: R−X→R−OH. This is a nucleophilic substitution...
CH3CH2OH can be converted into CH3CHO by ______________. (i) catalytic hydrogenation (ii) treatment with LiAlH4 (iii) treatment with pyridinium chlorochromate (iv) treatment with KMnO4
Option (iii) is the answer. Pyridinium chlorochromate (PCC), a combination of chromium trioxide, pyridine, and HCl, produces a high yield of aldehydes while preventing carboxylic acid oxidation....
What is the correct order of reactivity of alcohols in the following reaction? R—OH + HCl →(ZnCl2) R—Cl + H2O (i) 1° > 2° > 3° (ii) 1° < 2° > 3° (iii) 3° > 2° > 1° (iv) 3° > 1° > 2°
Option (iii) is the answer. The Lucas reagent is a combination of concentrated HCl and dry anhydrous $ZnCl_2$, and the reaction is known as the Lucas test. It's used to figure out whether a...
How many alcohols with molecular formula C4H10O are chiral? (i) 1 (ii) 2 (iii) 3 (iv) 4
Option (i) is the answer. Alcohols with the chemical formula $C_4H_{10}O$ can have a variety of structures. A similar chemical formula may be used to make a total of four alcohols. Butan-1-ol,...
Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields. (i) o-Cresol (ii) m-Cresol (iii) 2, 4-Dihydroxytoluene (iv) Benzyl alcohol
Option (iv) is the answer. Explanation: In the presence of light, halogenation follows the free radical pathway, and therefore reacts with the alkyl group to produce halo alkyl. In the presence of...
What is the structure and IUPAC name of glycerol?
Glycerol, commonly known as glycerin, is a triol molecule found in both plant and animal lipids. It's a substance that's utilised in dermatological treatments. It has mostly been utilised as a...
Write the IUPAC name of the following compounds.
(A)The compound's IUPAC name is 3-Ethyl-5-methyl hexane-2,4-diol. (B) The compound's IUPAC name is 1-Methoxy-3-nitrocyclohexane
Write the IUPAC name of the compound given below.
The compound's IUPAC name is 3-Methylpent-2-ene-1,2-diol. In the structure shown, the longest carbon atom chain is 5. Except for one double bond between C2 and C3 atoms, all carbon atom bonds are...
Name the factors responsible for the solubility of alcohols in water.
The following factors influence the solubility of alcohols in water: (i)Hydrogen bonding (ii)The size of the alkyl or aryl groups is the second factor to consider. (iii)The molecular mass of...
What is denatured alcohol?
Alcohols used for drinking are rendered unsuitable for human consumption by combining them with copper sulphate and pyridine, which give the liquid a yellow colour and a terrible odour,...
Suggest a reagent for the following conversion.
The oxidation of secondary alcohol into a ketone is seen in the chemical process above. Using oxidising agents such as chromic anhydride (CrO3), Pyridinium chlorochromate (PCC), and others, this is...
Out of 2-chloroethanol and ethanol which is more acidic and why?
Because of the presence of chlorine, which is an electron-withdrawing group, 2-chloroethanol is more acidic. The electron density in the –O-H bond decreases as a result of the negative inductive...
Suggest a reagent for conversion of ethanol to ethanal.
As reagents, PCC or Pyridinium chlorochromate might be employed. The oxidation of primary alcohol to an aldehyde is seen in the diagram above.
Suggest a reagent for conversion of ethanol to ethanoic acid.
For the afore mentioned reaction, acidified KMnO4 might be employed as a reagent. The oxidation of primary alcohol to a carboxylic acid is seen in the diagram above.
Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
O-nitrophenol is more volatile due to intramolecular hydrogen bonding between the NO2 and OH groups.
Out of o-nitrophenol and o-cresol which is more acidic?
In ortho-nitrophenol, there is an electron-withdrawing group NO2 in the ortho position, which increases the acidic strength and makes it more acidic. There is an electron releasing group in o-cresol...
When phenol is treated with bromine water, a white precipitate is obtained. Give the structure and the name of the compound formed.
2,4,6-tribromophenol is the name of the chemical produced in this process. The compound's structure is as follows:
Arrange the following compounds in increasing order of acidity and give a suitable explanation. Phenol, o-nitrophenol, o-cresol
o-cresol < Phenol < o-nitrophenol. NO2 o-nitrophenol becomes more acidic due to the presence of the electron-withdrawing group. The electron-releasing group in remaining reduces the acidic...
Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols
The following is a list of sodium metal's reactivity to alcohols in decreasing order: Primary alcohols are followed by secondary alcohols and finally tertiary alcohols. Because of two factors,...
What happens when benzene diazonium chloride is heated with water?
When benzene diazonium chloride is heated with water, it produces phenol as well as nitrogen gas and hydrochloric acid as by-products.
Arrange the following compounds in decreasing order of acidity. H2O, ROH, HC ≡ CH
The acidity of the following substances is listed in decreasing order: H2O > ROH > HC ≡ CH Because the carbon atoms here are sp hybridised, the electron density on the carbon atom is greater,...
Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
The enzymes involved in the fermentation of sucrose to produce ethanol are known as invertase and zymase. Sucrose is converted to glucose and fructose by invertase. The glucose and fructose are then...
How can propane-2-one be converted into tert- butyl alcohol?
Propane-2-one is hydrolyzed after being treated with CH3MgBr in the presence of dry ether (Grignard reagent).
Write the structures of the isomers of alcohols with molecular formula C4H10O. Which of these exhibits optical activity?
Because Butan-2-ol possesses a chiral carbon atom, it is the only one that displays optical activity.
Explain why the OH group in phenols is more strongly held as compared to OH group in alcohols
The phenol –OH group is immediately linked to the benzene ring's sp2hybridized carbon atom. The carbon-oxygen bond length in phenol is shorter than that in alkyl alcohol, which is related to the...
Explain why nucleophilic substitution reactions are not very common in phenols.
The ortho- and para-positions of the benzene ring become electron-rich as a result of the resonance, activating it for electrophilic substitution reactions. As a result, nucleophilic substitution...
Preparation of alcohols from alkenes involves the electrophilic attack on an alkene carbon atom. Explain its mechanism.
Step 1: Alkene protonation and production of a carbocation Step 2: Water's nucleophilic assault Step 3: Deprotonation occurs, resulting in the formation of alcohol. H30+ is now...
Explain why is O=C=O nonpolar while R—O—R is polar.
The dipole moments of the two C=O bonds are exactly equal and opposite, making O=C=O nonpolar. As a result, they cancel each other out, resulting in a net dipole moment of zero for O=C=O. Because...
Why is the reactivity of all the three classes of alcohols with conc. HCl and ZnCl2 (Lucas reagent) different?
This is due to the alkyl group's steric barrier and the carbocation's stability. Because the 1° carbocation is the least stable, primary alcohol has no reaction at ambient temperature. At ambient...
Write steps to carry out the conversion of phenol to aspirin.
Acetylsalicylic acid is another name for aspirin. The phenoxide ion is created by treating phenol with NaOH. The phenoxide ion is subsequently electrophilically substituted with CO2 to produce...
Nitration is an example of aromatic electrophilic substitution and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
The presence of the hydroxyl group in phenol causes it to be more nitrated. The ortho- and para-positions in the benzene ring become electron-rich due to the resonance effect induced by the –OH...
In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Because phenoxide ion is more reactive towards electrophilic aromatic substitution than phenol, phenoxide ion is treated with carbon dioxide (a weak electrophile) in Kolbe's process.
The dipole moment of phenol is smaller than that of methanol. Why?
The dipole moment of phenol is smaller than that of methanol due to the electron-withdrawing effect of the phenyl ring. Due to the resonance, the polarity of the C-O bond in phenol decreases.
Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
Williamson synthesis, in which an alkyl halide is reacted with sodium alkoxide, can be used to make ethers. This technique cannot be used to make di-tert-butyl ether because elimination takes...
Why is the C—O—H bond angle in alcohols slightly less than the tetrahedral angle whereas the C—O—C bond angle in ether is slightly greater?
Due to repulsion between the unshared pair of electrons or the lone pair of electrons on the oxygen atom, the C—O—H bond angle in alcohols is somewhat smaller than the tetrahedral angle.
Explain why low molecular mass alcohols are soluble in water.
This is due to the presence of intermolecular hydrogen bonding between alcohol molecules due to the presence of the OH group. The impact of the polar character of the –OH group of alcohol is...
Explain why p-nitrophenol is more acidic than phenol.
Because of the presence of an electron-withdrawing group, the -NO2 group, which improves the acidic strength of the molecule by stabilising the phenoxide ion, para-nitrophenol is more acidic than...
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Because alcohols contain intermolecular hydrogen bonding, their boiling temperatures differ from those of ethers of comparable molecular mass.
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Reason: (i) The carbon-oxygen bond obtains a partial double bond character due to the resonance. As a result, the carbon-oxygen bond in phenol shrinks in size. ii) Oxygen is directly connected to a...
Arrange water, ethanol and phenol in increasing order of acidity and give a reason for your answer.
Ethanol water phenol ethanol ethanol ethanol ethanol ethanol ethanol ethanol ethanol Because it forms the phenoxide ion after deprotonation and is stabilized by resonance, phenol is more acidic....
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of 2 ns and the number of photons emitted during the pulse source is 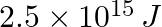 , calculate the energy of the source.
, calculate the energy of the source.
Frequency of radiation $\nu$ $\nu =\frac{1}{2.0\times 10^{-9}s}$ $\nu =5.0\times 10^{8}s^{-1}$ Energy (E) of source = Nhν Where, N is the no. photons emitted h is Planck’s constant ν denotes the...
The ejection of the photoelectron from the silver metal in the photoelectric effect experiment can be stopped by applying the voltage of 0.35 V when the radiation 256.7 nm is used. Calculate the work function for silver metal.
The energy associated with an incident photon (E) must equal the sum of its kinetic energy and the work function (W0) of the radiation, according to the rule of conservation of energy. E = W0 + K.E...
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 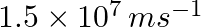 , calculate the energy with which it is bound to the nucleus.
, calculate the energy with which it is bound to the nucleus.
Energy of incident photon (E) is given by, $E=\frac{hc}{\lambda }$ $E=\frac{(6.626\times 10^{-34})(3\times 10^{8})}{(150\times 10^{-12})}=1.3252\times 10^{-15}\, J$ $\simeq 13.252\times 10^{-16}J$...
Emission transitions in the Paschen series end at orbit n = 3 and start from orbit n and can be represented as v =  (Hz) [1/3^2 – 1/n^ 2 ] Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
(Hz) [1/3^2 – 1/n^ 2 ] Calculate the value of n if the transition is observed at 1285 nm. Find the region of the spectrum.
Wavelength of the transition = 1285 nm =$1285 \times 10^{-9} m$(Given) $\nu =3.29\times 10^{15}(\frac{1}{3^{2}}-\frac{1}{n^{2}})$ Since$ \nu =\frac{c}{\lambda }$ =$\frac{3\times...
Calculate the wavelength for the emission transition if it starts from the orbit having radius 1.3225 nm and ends at 211.6 pm. Name the series to which this transition belongs and the region of the spectrum.
The radius of the n th orbit of hydrogen-like particles is given by, $r=\frac{0.529n^{2}}{Z}$ $r=\frac{5.29n^{2}}{Z}pm$ For radius (r1) = 1.3225 nm $=1.32225\times 10^{-9}m$ $=1322.25\times...
Dual behaviour of matter proposed by de Broglie led to the discovery of electron microscope often used for the highly magnified images of biological molecules and another type of material. If the velocity of the electron in this microscope is 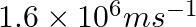 , calculate de Broglie wavelength associated with this electron.
, calculate de Broglie wavelength associated with this electron.
As per de Broglie’s equation, $\lambda =\frac{h}{m\nu }$ =$\frac{(6.626\times 10^{-34})}{9.103939\times 10^{-31}kg(1.6\times 10^{6}ms^{-1})}$ =$4.55\times 10^{-10}m\lambda =455pm$ Therefore, de...
Similar to electron diffraction, neutron diffraction microscope is also used for the determination of the structure of molecules. If the wavelength used here is 800 pm, calculate the characteristic velocity associated with the neutron.
From de Broglie’s equation, $\lambda =\frac{h}{m\nu }$ $\nu=\frac{h}{m\lambda}$ Where, v denotes the velocity of the neutron h is Planck’s constant m is the mass of the neutron λ is the wavelength...
If the velocity of the electron in Bohr’s first orbit is 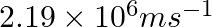 , calculate the de Broglie wavelength associated with it.
, calculate the de Broglie wavelength associated with it.
As per de Broglie’s equation, $\lambda =\frac{h}{m\nu}$ Where, λ is the wavelength of the electron h is Planck’s constant m is the mass of the electron v denotes the velocity of electron...
The velocity associated with a proton moving in a potential difference of 1000 V is 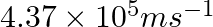 . If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
. If the hockey ball of mass 0.1 kg is moving with this velocity, calculate the wavelength associated with this velocity.
As per de Broglie’s expression, $\lambda =\frac{h}{m\nu }$ $ =\frac{(6.626\times 10^{-34})}{0.1kg(4.37\times 10^{5}ms^{-1})}$=$1.516\times 10^{-38}m$
If the position of the electron is measured within an accuracy of ± 0.002 nm, calculate the uncertainty in the momentum of the electron. Suppose the momentum of the electron is h/4πm × 0.05 nm, is there any problem in defining this value.
As per Heisenberg’s uncertainty principle, ∆x.∆p >= h/4π Where, ∆x = uncertainty in the position of the electron ∆p = uncertainty in the momentum of the electron Substituting the given values in...
The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. If any of these combination(s) has/have the same energy lists: n = 4, l = 2, ml = –2 , ms = –1/2 n = 3, l = 2, ml= 1 , ms = +1/2 n = 4, l = 1, ml = 0 , ms = +1/2 n = 3, l = 2, ml = –2 , ms = –1/2 n = 3, l = 1, ml = –1 , ms= +1/2 n = 4, l = 1, ml = 0 , ms = +1/2
The 4d, 3d, 4p, 3d, 3p, and 4p orbitals are home to electrons 1, 2, 3, 4, 5, and 6. (respectively). Ranking these orbitals in the increasing order of energies: (3p) < (3d) < (4p) < (4d).
The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electron experiences the lowest effective nuclear charge?
The nuclear charge that electrons (which are present in atoms containing multiple electrons) feel is determined by the distance between their orbital and the atom's nucleus. The smaller the...
Among the following pairs of orbitals which orbital will experience the larger effective nuclear charge? (i) 2s and 3s, (ii) 4d and 4f, (iii) 3d and 3p
The net positive charge acting on an electron in an atom's orbital with more than one electron is known as the nuclear charge. The distance between the orbital and the nucleus is inversely...
The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will experience more effective nuclear charge from the nucleus?
The net positive charge acting on an electron in an atom's orbital with more than one electron is known as the nuclear charge. The nuclear charge increases as the atomic number increases. Silicon...
Indicate the number of unpaired electrons in: (a)P (b)Si (c)Cr (d)Fe (e)Kr
(a)Phosphorus (P): The atomic number of phosphorus is 15 Electronic configuration of Phosphorus: 1s 2 2s 2 2p 6 3s 2 3p 3 This can be represented as follows: From the diagram, it can be observed...
(a) How many sub-shells are associated with n = 4? (b) How many electrons will be present in the sub-shells having ms value of –1/2 for n = 4?
(a)n = 4 (Given) For some value of ‘n’, the values of ‘l’ range from 0 to (n – 1). Here, the possible values of l are 0, 1, 2, and 3 Therefore, a total of 4 subshells are possible when n=4: the s,...
For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odour, flies and recycling of wastes for a good product.
To avoid odours and insects, the compost pit should be covered. To avoid interfering with the breakdown of the wastes, non-biodegradable wastes should not be thrown into the compost pit. They should...
How can domestic waste be used as manure?
To begin, trash must be separated into biodegradable and non-biodegradable categories. Biodegradable materials, such as leaves and food wastes, are deposited in landfills alongside microorganisms...
A large number of fish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill.
Bacteria eat phytoplankton, and this process necessitates the presence of dissolved oxygen. As a result, the greater the number of Phytoplankton present, the greater the consumption of dissolved...
What would have happened if the greenhouse gases were totally missing in the earth’s atmosphere? Discuss.
The greenhouse gases in our atmosphere capture the sun's UV rays and heat up the earth. Without greenhouse gases, the earth will be unable to retain any heat, which is necessary for the existence of...
What do you mean by green chemistry? How will it help decrease environmental pollution?
The manufacturing process employs our current understanding of chemistry principles to produce, develop, and deploy chemical compounds and products that reduce the amount of harmful chemicals in the...
What are pesticides and herbicides? Explain giving examples.
A pesticide is a combination of two or more chemicals that is used to kill bugs. Plant diseases, weeds, insects, mollusks, and other pests that damage plants and cause them to die must all be...
Do you observe any soil pollution in your neighbourhood? What efforts will you make for controlling the soil pollution?
Pesticides and fertilisers are the most common contaminants that pollute soil. Because pesticides like DDT are not soluble in water, they remain in the soil for prolonged periods of time, polluting...
What do you mean by Biochemical Oxygen Demand (BOD)?
The quantity of oxygen required by bacteria to breakdown organic materials in a given volume of water is known as biochemical oxygen demand. A BOD of less than 5 ppm indicates clean water, but a BOD...
Have you ever observed any water pollution in your area? What measures would you suggest to control it?
Water pollution is caused by human activities such as storm-water drainage, run-off from agricultural areas, wastewater treatment plant emissions, and so on. Industries and factories emit toxic...
What are the major causes of water pollution? Explain.
Water pollution happens when undesired and unwanted chemicals are introduced into water bodies, suffocating the aquatic life that lives there. The following are the primary sources of water...
What do you mean by ozone hole? What are its consequences?
Polar stratospheric clouds offer a surface for the reaction of hypochlorous acid and chlorine nitrate, which produces molecular chlorine after further reaction. Photolysis of HOCl and Molecular...
What are the reactions involved for ozone layer depletion in the stratosphere?
In the stratosphere, ozone is actually formed by the action of UV rays on Dioxygen molecules(O2). (i) O2(g) →UV O(g) +O(g) (ii) O2(g) + O(g) ↔UV O3(g) The second reaction demonstrates that the...
What are the harmful effects of photochemical smog and how can they be controlled?
Photochemical smog is oxidising in nature because it contains NO2 and O3, which cause rubber, stones, metals, and painted surfaces to corrode. Formaldehyde, PAN, and acrolein are also found in...
Write down the reactions involved during the formation of photochemical smog.
The interaction of sunlight with nitrogen oxides and hydrocarbons produces photochemical smog. The major components of photochemical smog include formaldehyde, nitric oxide, ozone, PAN, and...
What is smog? How is classical smog different from photochemical smogs?
Smog is a combination of smoke and fog which causes air pollution. There are two types of smog: a) Photochemical smog b) Classical smog They can be differentiated as follows: Photochemical smog ...
Statues and monuments in India are affected by acid rain. How?
By interacting with water in the presence of ambient oxygen, nitrogen oxides (NO2, NO) and sulphur oxides (SO2 and SO3) produced by the combustion of coal, car gasoline, and other fossil fuels...
List gases which are responsible for greenhouse effect.
The major gases that cause greenhouse effect are: 1) Chlorofluorocarbons (CFCs) 2) Methane (CH4) 3) Carbon dioxide (CO2) 4) Nitrous oxide (NO) 5) Water(H2O) 6) Ozone (O3)
Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Carbon dioxide (CO2) and carbon monoxide (CO) are both produced when various fuels are burned. In nature, carbon monoxide is harmful, but carbon dioxide is non-toxic. Because carbon monoxide forms a...
Explain tropospheric pollution in 100 words.
Tropospheric pollution is caused by the presence of undesirable chemicals in the troposphere's lowest layer. Nitrogen oxides, sulphur oxides, carbon, and hydrocarbons are the most common...
What is the maximum number of emission lines when the excited electron of a H atom in n = 6 drops to the ground state?
A total number of 15 lines (5 + 4 + 3 + 2 + 1) will be obtained in this hydrogen emission spectrum. The total number of spectral lines emitted when an electron drops to the ground state from the...
How much energy is required to ionise a H atom if the electron occupies n = 5 orbit? Compare your answer with the ionization enthalpy of H atom (energy required to remove the electron from n =1 orbit).
The expression for the ionization energy is given by, $E_{n} =\frac{-(2.18\times 10^{-18})Z^{2}}{n^{2}}$ Where Z denotes the atomic number and n is the principal quantum number For the ionization...
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes the transition from an energy level with n = 4 to an energy level with n = 2?
The $n_{i} = 4$ to$ n_{f}$= 2 transition results in a spectral line of the Balmer series. The energy involved in this transition can be calculated using the following expression: $E=2.18\times...
Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength 6800 Å. Calculate threshold frequency (ν0 ) and work function (W0 ) of the metal.
Threshold wavelength of the radiation $(\lambda _{0})= 6800 Å=6800\times 10^{-10}\, m$ Threshold frequency of the metal $(\nu _{0}) =\frac{c}{\lambda _{0}}=$ $\frac{3\times 10^{8}ms^{-1}}{6.8\times...
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionise the sodium atom. Calculate the ionisation energy of sodium in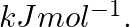
Ionization energy (E) of sodium =$ \frac{N_{A}hc}{\lambda }$ =$\frac{(6.023\times 10^{23}\, mol^{-1})(6.626\times 10^{-34})Js(3\times 10^{8})ms^{-1}}{242\times 10^{-9}m}$ =$4.947\times 10^{5}\, J\,...
A photon of wavelength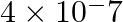 m strikes on metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron
m strikes on metal surface, the work function of the metal is 2.13 eV. Calculate (i) the energy of the photon (eV), (ii) the kinetic energy of the emission, and (iii) the velocity of the photoelectron 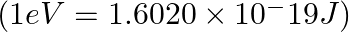 .
.
(i) Energy of the photon $(E)= h\nu =\frac{hc}{\lambda }$ Where, h denotes Planck’s constant, whose value is $6.626\times 10^{-34}\,Js$ c denotes the speed of light =$ 3\times 10^{8}\,m/s$...
What is the number of photons of light with a wavelength of 4000 pm that provides 1J of energy?
Energy of one photon (E) =$ h\nu$ Energy of ‘n’ photons $E_{n} = nh\nu \Rightarrow n=\frac{E_{n}\lambda }$ Where, \lambdaλ is the wavelength of the photons = 4000 pm = $4000\times 10^{-12}\, m$ c...
Calculate the wavelength, frequency and wavenumber of a light wave whose period is 2.0 × 10–10 s.
Frequency of the light wave $\nu$ = $\frac{1}{Period} \frac{1}{Period}$ $=\frac{1}{2.0\times 10^{-10}\, s} =5.0\times 10^{9}\, s^{-1 }$ Wavelength of the light wave$\lambda=c\nu$ Where, c denotes...
Find the energy of each of the photons which (i) correspond to light of frequency 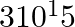 Hz. (ii) have a wavelength of 0.50 Å.
Hz. (ii) have a wavelength of 0.50 Å.
(i) The energy of a photon (E) can be calculated by using the following expression: $E= h\nu$ Where, ‘h’ denotes Planck’s constant, which is equal to $6.626\times 10^{-34}\, Js\nuν$ (frequency of...
Yellow light emitted from a sodium lamp has a wavelength (λ) of 580 nm. Calculate the frequency (ν) and wavenumber (ν ) of the yellow light.
Rearranging the expression, $\lambda =\frac{c}{\nu }$ the following expression can be obtained, $\nu =\frac{c}{ \lambda }$ ……….(1) Here, $\nu$ denotes the frequency of the yellow light c denotes...
Write the complete symbol for the atom with the given atomic number (Z) and atomic mass (A) (I)Z = 17, A = 35 (II)Z = 92, A = 233 (III)Z = 4, A = 9
(i)\({}_{17}^{35}C\) (ii)\({}_{92}^{233}C\) (iii)\({}_{4}^{9}C\)
How many neutrons and protons are there in the following nuclei? 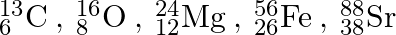
\({}_{6}^{13}C\): Mass number of carbon-13 = 13 Atomic number of carbon = Number of protons in one carbon atom = 6 Therfore, total number of neutrons in 1 carbon atom = Mass number – Atomic number =...
(i) Calculate the total number of electrons present in one mole of methane. (ii) Find (a) the total number and (b) the total mass of neutrons in 7 mg of 14C. (Assume that mass of a neutron = 1.675 × 10–27 kg). (iii) Find (a) the total number and (b) the total mass of protons in 34 mg of NH3 at STP. Will the answer change if the temperature and pressure are changed?
(i) 1 molecule of methane contains 10 electrons (6 from carbon, 4 from hydrogen) Therefore, 1 mole of methane contains 10*NA = 6.022*1024 electrons. (ii) Number of neutrons in 14g (1 mol) of 14C =...
(i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
1 electron weighs 9.109*10-31 kg. Therefore, number of electrons that weigh 1 g (10-3 kg) = 1.098*1027 electrons (ii) Mass of one mole of electrons = NA* mass of one electron =...
Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation: N2 (g) + H2(g)→ 2NH3 (g) (i) Calculate the mass of 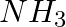 produced if
produced if 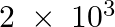 g
g 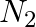 reacts with
reacts with 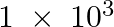 g of
g of 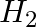 ? (ii) Will any of the two reactants remain unreacted? (iii) If yes, which one and what would be its mass.
? (ii) Will any of the two reactants remain unreacted? (iii) If yes, which one and what would be its mass.
(i) Balance the given equation: $N_{ 2 }\;(g) \; + \; 3H_{ 2 } \;(g) \; \rightarrow \; 2NH_{ 3 }\;(g) $ Thus, 1 mole (28 g) of N2 reacts with 3 mole (6 g) of H2 to give 2 mole (34 g)...
How are 0.50 mol 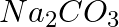 and 0.50 M
and 0.50 M 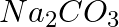 different?
different?
Molar mass of $Na_{ 2 }CO_{ 3 }$ = (2 × 23) + 12 + (3 × 16) = $106 g mol^{ -1 }$ 1 mole of $Na_{ 2 }CO_{ 3 }$ means 106 g of $Na_{ 2 }CO_{ 3 }$ Therefore, 0.5 mol of $Na_{ 2 }CO_{ 3 }$...
If 10 volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour would be produced?
2H2(g) +O2(g) →2H2O(g) 2 volumes of dihydrogen react with 1 volume of dioxygen to produce two volumes of vapour. Hence, 10 volumes of dihydrogen will react with five volumes of dioxygen to...
Convert the following into basic units: (i) 28.7 pm (ii) 15.15 pm (iii) 25365 mg
(i) 28.7 pm $1 pm = 10^{ -12 } \; m$ $28.7 pm = 28.7 \times 10^{ -12 } \; m$ $= 2.87 \times 10^{ -11 } \; m$ (ii) 15.15 pm $1 pm = 10^{ -12 } \; m$ $15.15 pm = 15.15 \times 10^{ -12 } \; m$...
Which one of the following will have the largest number of atoms? (i) 1 g Au (s) (ii) 1 g Na (s) (iii) 1 g Li (s) (iv) 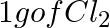 (g)
(g)
(i) 1 g Au (s) = $\frac{ 1 }{ 197 }$ mol of Au (s) = $\frac{ 6.022 \; \times \; 10^{ 23 } }{ 197 }$ atoms of Au (s) = $3.06 \times \; 10^{ 21 }$ atoms of Au (s) (ii) 1 g Na (s) = $\frac{ 1...
Calculate the molarity of a solution of ethanol in water, in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
Mole fraction of $C_{ 2 }H_{ 5 }OH$ = $\frac{Number \; of \; moles \; of \; C_{ 2 }H_{ 5 }OH}{Number \; of \; moles \; of \; solution}$ $0.040 = \frac{n_{C_{ 2 }H_{ 5 }OH}}{n_{C_{ 2 }H_{ 5 }OH} \; +...
What will be the mass of one 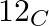 atom in g?
atom in g?
1 mole of carbon atoms =$ 6.023 \; \times \; 10^{ 23 }$atoms of carbon = 12 g of carbon Therefore, mass of $1 _{}^{ 12 }\textrm{ C }$ atom = $\frac{ 12 \; g }{ 6.022 \; \times \; 10^{ 23 }}$...
How many significant figures should be present in the answer of the following calculations? (i)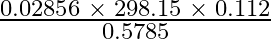 (ii) 5 × 5.365 (iii) 0.012 + 0.7864 + 0.0215
(ii) 5 × 5.365 (iii) 0.012 + 0.7864 + 0.0215
(i) $\frac{ 0.02856 \; \times \; 298.15 \; \times \; 0.112}{ 0.5785 }$ Least precise no. of calculation = 0.112 Therefore, no. of significant numbers in the answer = No. of significant numbers in...
Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
Molar mass of Argon: =$ [( 35.96755 \; \times \; \frac{ 0.337 }{ 100 }) + ( 37.96272 \; \times \; \frac{ 0.063 }{ 100 })+ ( 39.9624 \; \times \; \frac{ 99.600 }{ 100 })]$ =$ [0.121 + 0.024 + 39.802]...
Calculate the number of atoms in each of the following (i) 52 moles of Ar (ii) 52 u of He (iii) 52 g of He
(i) 52 moles of Ar 1 mole of Ar = $6.023 \; \times \; 10^{ 23 }$atoms of Ar Therefore, 52 mol of Ar = 52 × $6.023 \; \times \; 10^{ 23 }$atoms of Ar = $3.131 \; \times \; 10^{ 25 }$ atoms of Ar...
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Find: (i) Empirical formula (ii) Molar mass of the gas, and (iii) Molecular formula
(i) Empirical formula 1 mole of $CO_{ 2 }$ contains 12 g of carbon Therefore, 3.38 g of $CO_{ 2 }$ will contain carbon = $\frac{ 12 \; g }{ 44 \; g } \; \times 3.38 \; g$ = 0.9217 g 18 g of...
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3 (s) + 2 HCl (aq) → CaCl2(aq) + CO2 (g) + H2O(l) What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
0.75 M of HCl ≡ 0.75 mol of HCl are present in 1 L of water ≡ $[(0.75 mol)\times(36.5 g mol–1 )]$ HCl is present in 1 L of water ≡ 27.375 g of HCl is present in 1 L of water Thus, 1000 mL of...
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction: 4 HCl (aq) + MnO2(s) → 2H2O (l) + MnCl2(aq) + Cl2 (g) How many grams of HCl react with 5.0 g of manganese dioxide?
1 mol of $MnO_{2}$ = 55 + 2 × 16 = 87 g 4 mol of HCl = 4 × 36.5 = 146 g 1 mol of $MnO_{2}$ reacts with 4 mol of HCl 5 g of $MnO_{ 2 }$will react with: =$ \frac{146 \; g}{87 \; g} \; \times \; 5...
In a reaction A + B2 → AB2 Identify the limiting reagent, if any, in the following reaction mixtures. (i) 300 atoms of A + 200 molecules of B (ii) 2 mol A + 3 mol B (iii) 100 atoms of A + 100 molecules of B (iv) 5 mol A + 2.5 mol B (v) 2.5 mol A + 5 mol B
Reagent limitation: It establishes the magnitude of a reaction. It is the first to be consumed in a reaction, causing the process to come to a halt and limiting the number of products produced. (i)...
If the speed of light is 3.0 × 10^8 m s^(–1), calculate the distance covered by light in 2.00 ns
Time taken = 2 ns = $2 \times10^{ -9 }$ s Now, Speed of light =$3 \times10^{ 8 } ms^{ -1 }$ So, Distance travelled in 2 ns = speed of light * time taken =$(3 \times10^{ 8 })(2 \times10^{ -9 })$ = $6...
The following data are obtained when dinitrogen and dioxygen react together to form different compounds:;(a) Which law of chemical combination is obeyed by the above experimental data? Give its statement. (b) Fill in the blanks in the following conversions: (i) 1 km = …………………. mm = …………………. pm (ii) 1 mg = …………………. kg = …………………. ng (iii) 1 mL = …………………. L = …………………. dm3
(a) If the mass of N2 is set at 28 g, the mass of O2 that will combine with it is 32 grammes, 64 grammes, 32 grammes, and 80 grammes. O2 has a mass-to-number ratio of 1: 2: 1: 5. As a result, the...
Round up the following upto three significant figures: (a) 34.216 (b) 10.4107 (c)0.04597 (d)2808
(a) The number after round up is: 34.2 (b) The number after round up is: 10.4 (c)The number after round up is: 0.0460 (d)The number after round up is: 2808
How many significant figures are present in the following? (a) 0.0027 (b) 209 (c) 6005 (d) 136,000 (e) 900.0 (f) 2.0035
(i) 0.0027: 2 significant numbers. (ii) 209: 3 significant numbers. (iii) 6005: 4 significant numbers. (iv) 136,000:3 significant numbers. (v) 900.0: 4 significant numbers. (vi) 2.0035: 5...
Express the following in the scientific notation: (i) 0.0048 (ii) 234,000 (iii) 8008 (iv) 500.0 (v) 6.0012
(a$) 0.0048= 4.8 \times10^{-3}$ (b) $234,000 = 2.34 \times10^{5}$ (c) $8008= 8.008 \times10^{3}$ (d) $500.0 = 5.000 \times10^{2}$ (e) $6.0012 = 6.0012 \times10^{0}$
